


1. Distigmine
2. Hexamarium
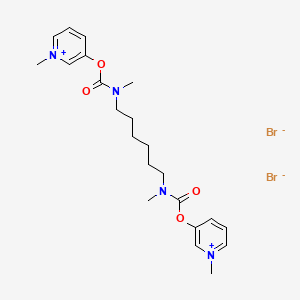
3. Pyridinium, 3,3'-(1,6-hexanediylbis((methylimino)carbonyl)oxy)bis-1-methyl-, Dibromide
4. Ubretid
1. 15876-67-2
2. Ubretid
3. Ubritil
4. Distigmini Bromidum
5. Hexamarium
6. Bromure De Distigmine
7. Bromuro De Distigmina
8. Distigminebromide
9. Hexamarium Bromide
10. Bc 51
11. Distigmine Dibromide
12. Bc-51
13. 750f36op6j
14. 3,3'-(1,6-hexanediylbis((methylimino)carbonyl)oxy)bis(1-methylpyridinium) Dibromide
15. (1-methylpyridin-1-ium-3-yl) N-methyl-n-[6-[methyl-(1-methylpyridin-1-ium-3-yl)oxycarbonylamino]hexyl]carbamate;dibromide
16. 3,3'-(((hexane-1,6-diylbis(methylazanediyl))bis(carbonyl))bis(oxy))bis(1-methylpyridin-1-ium) Bromide
17. 3-hydroxy-1-methylpyridinium Bromide Hexamethylenebis-(n-methylcarbamate)
18. (1-methylpyridin-1-ium-3-yl) N-methyl-n-[6-[methyl-(1-methylpyridin-1-ium-3-yl)oxycarbonylamino]hexyl]carbamate,dibromide
19. 3,3'-{hexane-1,6-diylbis[(methylcarbamoyl)oxy]}bis(1-methylpyridin-1-ium) Dibromide
20. Distigmini Bromidum [inn-latin]
21. Distigmine Bromide [inn:ban:jan]
22. Bromure De Distigmine [inn-french]
23. Einecs 240-013-0
24. Bromuro De Distigmina [inn-spanish]
25. Adcostigmine
26. Unii-750f36op6j
27. Ubretid (tn)
28. 3-hydroxy-1-methylpyridinium Bromide Hexamethylenebis(methylcarbamate)
29. Schembl148842
30. Distigmine Bromide [mi]
31. Chembl1098285
32. Distigmine Bromide [inn]
33. Distigmine Bromide [jan]
34. Chebi:31512
35. Distigmine Bromide (jp17/inn)
36. Dtxsid60935985
37. Distigmine Bromide [mart.]
38. Bcp16582
39. Distigmine Bromide [who-dd]
40. Mfcd00867188
41. Akos015914117
42. Pyridinium, 3-hydroxy-1-methyl-, Bromide, Hexamethylenebis(methylcarbamate)
43. Ac-8868
44. Pyridinium, 3,3'-(1,6-hexanediylbis((methylimino)carbonyl)oxy)bis-1-methyl-, Dibromide
45. As-74357
46. Sy226319
47. Hy-119577
48. Cs-0070000
49. Ft-0718457
50. C72992
51. D01228
52. A855351
53. Q3277473
54. 3,3'-(1,6-hexanediylbis((methylimino)carbonyl)oxy)bis(1-methylpyridinium)dibromide
55. 3,3'-{hexane-1,6-diylbis[(methylcarbamoyl)oxy]}bis(1-methylpyridinium) Dibromide
56. Hexamethylenebis(n-methylcarbaminoyl-1-methyl-3-hydroxypyridinium Bromide)
57. 1-methyl-3-{[methyl({6-[methyl({[(1-methylpyridin-1-ium-3-yl)oxy]carbonyl})amino]hexyl})carbamoyl]oxy}pyridin-1-ium Dibromide
58. 3,3-(((hexane-1,6-diylbis(methylazanediyl))bis(carbonyl))bis(oxy))bis(1-methylpyridin-1-ium) Bromide
59. Pyridinium, 3,3'-(1,6-hexanediylbis((methylimino)carbonyl)oxy)bis(1-methyl-, Bromide (1:2)
| Molecular Weight | 576.3 g/mol |
|---|---|
| Molecular Formula | C22H32Br2N4O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | 576.07698 g/mol |
| Monoisotopic Mass | 574.07903 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)