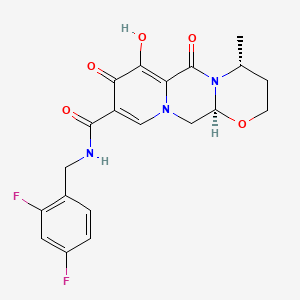

1. (4r,12as)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
2. (4r,9as)-5-hydroxy-4-methyl-6,10-dioxo-3,4,6,9,9a,10-hexahydro-2h-1-oxa-4a,8a-diaza-anthracene-7-carboxylic Acid- 2,4 Difluorobenzylamide
3. (4s,12ar)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
4. (4s,12as)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
5. Dolutegravir S,r Isomer
6. Dolutegravir S,s-isomer
7. Dolutegravir Sodium
8. Dolutegravir Sodium Monohydrate
9. Gsk 1349572a
10. Gsk-1349572
11. Gsk-1349572a
12. Gsk1349572a
13. S-gsk1349572
14. Tivicay
15. Tivicay Pd
1. 1051375-16-6
2. Gsk1349572
3. S/gsk1349572
4. Tivicay
5. Gsk-1349572
6. Gsk 1349572
7. Dolutegravir (gsk1349572)
8. S-349572
9. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
10. Dolutegravir Dtg
11. Dolutegravir [usan]
12. Chebi:76010
13. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
14. Dko1w9h7m1
15. Dolutegravir (usan)
16. 1051375-16-6 (free)
17. Tivicay (tn)
18. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.0^{3,8}]tetradeca-10,13-diene-13-carboxamide
19. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.03,8]tetradeca-10,13-diene-13-carboxamide
20. S-gsk1349572
21. Dolutegravir [usan:inn]
22. Unii-dko1w9h7m1
23. Soltegravir
24. Hsdb 8152
25. 3s3m
26. 3s3n
27. 3s3o
28. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2h-pyrido[[?]:[?]]pyrazino[[?]][1,3]oxazine-9-carboxamide
29. Dolutegravir [mi]
30. Dolutegravir [inn]
31. Dolutegravir [vandf]
32. Schembl82071
33. Mls006011137
34. Dolutegravir [who-dd]
35. Gtpl7365
36. Chembl1229211
37. Dtxsid90909356
38. Ex-a1695
39. Bdbm50062551
40. Mfcd20488027
41. S2667
42. Zinc58581064
43. Akos025396657
44. S/gsk-1349572
45. Bcp9000620
46. Ccg-268876
47. Cs-0454
48. Db08930
49. Ncgc00346629-01
50. Ncgc00346629-02
51. 2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide, N-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-, (4r,12as)-
52. Ac-28371
53. As-75277
54. Hy-13238
55. Smr004702915
56. S/gsk1349572,gsk1349572
57. D10066
58. A854801
59. Q937224
60. J-501471
61. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.0(3),?]tetradeca-10,13-diene-13-carboxamide
62. (4r,12.alpha.s)-n-((2,4-difluorophenyl)methyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12.alpha.-hexahydro-2h-pyrido(1',2':4,5)pyrazino(2,1-.beta.)(1,3)oxazine-9-carboxamide
63. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-[1,3]oxazino[3,2-a]pyrido[1,2-d]pyrazine-9-carboxamide
64. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxamide
65. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1,2:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
66. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1 ,3]oxazine-9-carboxamide
67. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2h-pyrido[5,6]pyrazino[2,6-b][1,3]oxazine-9-carboxamide
| Molecular Weight | 419.4 g/mol |
|---|---|
| Molecular Formula | C20H19F2N3O5 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 419.12927704 g/mol |
| Monoisotopic Mass | 419.12927704 g/mol |
| Topological Polar Surface Area | 99.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 829 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
HIV Integrase Inhibitors
National Library of Medicine's Medical Subject Headings. Dolutegravir. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
The recommended dose of TIVICAY in pediatric patients aged 12 years and older and weighing at least 40 kg is 50 mg administered orally once daily. If efavirenz, fosamprenavir/ritonavir, tipranavir/ritonavir, or rifampin are coadministered, the recommended dose of TIVICAY is 50 mg twice daily. Safety and efficacy of TIVICAY have not been established in pediatric patients younger than 12 years or weighing less than 40 kg, or in pediatric patients who are INSTI-experienced with documented or clinically suspected resistance to other INSTIs (raltegravir, elvitegravir).
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
TIVICAY (dolutegravir) is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and children aged 12 years and older and weighing at least 40 kg. The following should be considered prior to initiating treatment with TIVICAY: Poor virologic response was observed in subjects treated with TIVICAY 50 mg twice daily with an integrase strand transfer inhibitor (INSTI)-resistance Q148 substitution plus 2 or more additional INSTI-resistance substitutions, including L74I/M, E138A/D/K/T, G140A/S, Y143H/R, E157Q, G163E/K/Q/R/S, or G193E/R.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Renal clearance of unchanged drug is a minor pathway of elimination for dolutegravir. In a trial comparing 8 subjects with severe renal impairment (CrCl <30 mL/min) with 8 matched healthy controls, AUC, Cmax, and C24 of dolutegravir were decreased by 40%, 23%, and 43%, respectively, compared with those in matched healthy subjects. The cause of this decrease is unknown. Population pharmacokinetic analysis using data from SAILING and VIKING-3 trials indicated that mild and moderate renal impairment had no clinically relevant effect on the exposure of dolutegravir. No dosage adjustment is necessary for treatment-naive or treatment-experienced and INSTI-naive patients with mild, moderate, or severe renal impairment or for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance) with mild or moderate renal impairment. Caution is warranted for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance [see Microbiology (12.4)]) with severe renal impairment, as the decrease in dolutegravir concentrations may result in loss of therapeutic effect and development of resistance to TIVICAY or other coadministered antiretroviral agents. Dolutegravir has not been studied in patients requiring dialysis.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is primarily metabolized and eliminated by the liver. In a trial comparing 8 subjects with moderate hepatic impairment (Child-Pugh Score B) with 8 matched healthy controls, exposure of dolutegravir from a single 50-mg dose was similar between the 2 groups. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh Score A or B). The effect of severe hepatic impairment (Child-Pugh Score C) on the pharmacokinetics of dolutegravir has not been studied. Therefore, TIVICAY is not recommended for use in patients with severe hepatic impairment.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. Studies in lactating rats and their offspring indicate that dolutegravir was present in rat milk. It is not known whether dolutegravir is excreted in human milk. Because of both the potential for HIV transmission and the potential for adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving TIVICAY.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Pregnancy Category B. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, and dolutegravir was shown to cross the placenta in animal studies, this drug should be used during pregnancy only if clearly needed.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir (TIVICAY) should not be used with etravirine without coadministration of atazanavir/ritonavir, darunavir/ritonavir, or lopinavir/ritonavir.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including TIVICAY. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment. Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barre syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
For more Drug Warnings (Complete) data for Dolutegravir (8 total), please visit the HSDB record page.
Dolutegravir is indicated in combination with other antiretroviral agents for the treatment of patients with HIV-1 infection that comply with the characteristics of being adults or children aged 12 years and older and present at least a weight of 40 kg. The FDA combination therapy approval of dolutegravir and rilpivirine is indicated for adults with HIV-1 infections whose virus is currently suppressed (< 50 copies/ml) on a stable regimen for at least six months, without history of treatment failure and no known substitutions associated to resistance to any of the two components of the therapy.
FDA Label
Tivicay is indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults, adolescents and children of at least 6 years of age or older and weighing at least 14 kg.
Tivicay is indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults, adolescents and children of at least 4 weeks of age or older and weighing at least 3 kg.
HIV-1 infected subjects on dolutegravir monotherapy demonstrated rapid and dose-dependent reduction of antiviral activity with declines of HIV-1 RNA copies per ml. The antiviral response was maintained for 3 to 4 days after the last dose. The sustained response obtained in clinical trials indicates that dolutegravir has a tight binding and longer dissociative half-life providing it a high barrier to resistance. The combination therapy (ripivirine and dolutegravir) presented the same viral suppression found in previous three-drug therapies without integrase strand transfer inhibitor mutations or rilpivirine resistance.
HIV Integrase Inhibitors
Inhibitors of HIV INTEGRASE, an enzyme required for integration of viral DNA into cellular DNA. (See all compounds classified as HIV Integrase Inhibitors.)
J05AX12
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AJ - Integrase inhibitors
J05AJ03 - Dolutegravir
Absorption
When 50 mg of dolutegravir once daily was orally administered to HIV-1 infected adults, the AUC, Cmax, and Cmin is 53.6 mcg h/mL, 3.67 mcg/mL, and 1.11 mcg/mL, respectively. The peak plasma concentration was observed 2 to 3 hours post-dose. Steady state is achieved within approximately 5 days with average accumulation ratios for AUC, Cmax, and C24h ranging from 1.2 to 1.5. When 50 mg once daily is given to pediatric patients (12 to < 18 years and weighing 40 kg) the Cmax, AUC, and C24 is 3.49 mcg/mL, 46 mcg.h/mL, and 0.90 mcg/mL respectively.
Route of Elimination
When a single oral dose of dolutegravir is given, nearly all complete dose is recovered in a proportion of 53% excreted unchanged in the feces and 31% excreted in urine. The renal eliminated recovered dose consists of ether glucuronide of dolutegravir (18.9%), a metabolite formed by oxidation at the benzylic carbon (3.0%), a hydrolytic N-dealkylation product (3.6%) and unchanged drug (< 1%).
Volume of Distribution
The administration of a dose of 50 mg of dolutegravir presents an apparent volume of distribution of 17.4 L. The median dolutegravir concentration in CSF was 18 ng/mL after 2 weeks of treatment.
Clearance
The apparent clearance rate of dultegravir is 1.0 L/h.
... After a single oral dose of [14C] dolutegravir, 53% of the total oral dose was excreted unchanged in feces. Thirty-one percent of the total oral dose was excreted in urine, represented by an ether glucuronide of dolutegravir (18.9% of total dose), a metabolite formed by oxidation at the benzylic carbon (3.0% of total dose), and its hydrolytic N-dealkylation product (3.6% of total dose). Renal elimination of unchanged drug was low (<1% of the dose).
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is highly bound (=98.9%) to human plasma proteins based on in vivo data and binding is independent of plasma concentration of dolutegravir. The apparent volume of distribution (Vd/F) following 50-mg once-daily administration is estimated at 17.4 L based on a population pharmacokinetic analysis.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Food increased the extent of absorption and slowed the rate of absorption of dolutegravir. Low-, moderate-, and high-fat meals increased dolutegravir AUC(0-8) by 33%, 41%, and 66%; increased Cmax by 46%, 52%, and 67%; and prolonged Tmax to 3, 4, and 5 hours from 2 hours under fasted conditions, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Following oral administration of dolutegravir, peak plasma concentrations were observed 2 to 3 hours postdose. With once-daily dosing, pharmacokinetic steady state is achieved within approximately 5 days with average accumulation ratios for AUC, Cmax, and C24 h ranging from 1.2 to 1.5. Dolutegravir plasma concentrations increased in a less than dose-proportional manner above 50 mg. Dolutegravir is a P-glycoprotein substrate in vitro. The absolute bioavailability of dolutegravir has not been established.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is highly metabolized through three main pathways and it forms no long-lived metabolites. The first pathway is defined by the glucuronidation by UGT1A1, the second pathway by carbon oxidation by CYP3A4 and the third pathway is what appears to be a sequential oxidative defluorination and glutathione conjugation. The main metabolite found in blood plasma is the ether glucuronide form (M2) and its chemical properties disrupt its ability to bind metal ions, therefore, it is inactive.
Dolutegravir is primarily metabolized via UGT1A1 with some contribution from CYP3A. ... ether glucuronide of dolutegravir (18.9% of total dose), a metabolite formed by oxidation at the benzylic carbon (3.0% of total dose), and its hydrolytic N-dealkylation product (3.6% of total dose). ...
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
The half-life of dolutegravir is 14 hours.
Dolutegravir has a terminal half-life of approximately 14 hours and an apparent clearance (CL/F) of 1.0 L/h based on population pharmacokinetic analyses.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is an HIV-1 antiviral agent. It inhibits HIV integrase by binding to the active site and blocking the strand transfer step of retroviral DNA integration in the host cell. The strand transfer step is essential in the HIV replication cycle and results in the inhibition of viral activity. Dolutegravir has a mean EC50 value of 0.5 nM (0.21 ng/mL) to 2.1 nM (0.85 ng/mL) in peripheral blood mononuclear cells (PBMCs) and MT-4 cells.
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle. Strand transfer biochemical assays using purified HIV-1 integrase and pre-processed substrate DNA resulted in IC50 values of 2.7 nM and 12.6 nM.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922