

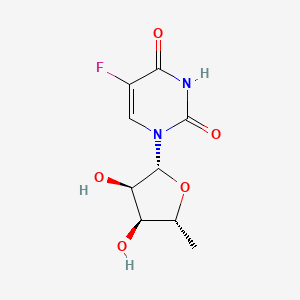

1. 5'-deoxy-5'-fluorouridine
2. 5'-deoxy-5-fluorouridine
3. 5'-dfur
4. 5'-fluoro-5'-deoxyuridine
5. Doxyfluridine
6. Furtulon
7. Ro 21-9738
1. 5'-deoxy-5-fluorouridine
2. 5-fluoro-5'-deoxyuridine
3. Furtulon
4. 3094-09-5
5. 5'-dfur
6. Flutron
7. Uridine, 5'-deoxy-5-fluoro-
8. Doxifluridina
9. Doxifluridinum
10. Fulturon
11. Ro 219738
12. Ro 21-9738
13. Capecitabine Related Compound B
14. 1-(beta-d-5-desoxyribofuranoxyl)-5-fluoruracil
15. Chembl1130
16. V1jk16y2jp
17. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoropyrimidine-2,4(1h,3h)-dione
18. Chebi:31521
19. Nsc-758890
20. Ncgc00093926-03
21. Ro-219738
22. Dsstox_cid_2967
23. 5'fdur
24. Dsstox_rid_76809
25. Dsstox_gsid_22967
26. Mfcd00866530
27. 5'-doxifluridine
28. 5'-dfurd
29. Furtulon (tn)
30. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methyl-tetrahydrofuran-2-yl]-5-fluoro-pyrimidine-2,4-dione
31. Smr000326811
32. Cas-3094-09-5
33. Uridine-5'-deoxy-5-fluoro-
34. 5'dfurd
35. Sr-01000075886
36. Doxifluridine [inn:jan]
37. 5-dfur
38. Doxifluridinum [inn-latin]
39. Doxifluridina [inn-spanish]
40. Einecs 221-440-1
41. 5-deoxy-5-fluorouridine
42. Doxifluridine [mi]
43. 5-fluoro-5`-deoxyuridine
44. Unii-v1jk16y2jp
45. Doxifluridine [inn]
46. Doxifluridine [jan]
47. Schembl8094
48. Doxifluridine (jp17/inn)
49. Lopac0_000537
50. Mls001332579
51. Mls001332580
52. Mls002172440
53. Mls002207077
54. Doxifluridine [mart.]
55. Doxifluridine [who-dd]
56. Dtxsid2022967
57. Hms2090c22
58. Hms2231i05
59. Hms3261l15
60. Amc 0101
61. Amy13408
62. Hy-b0021
63. Zinc1319177
64. Tox21_111231
65. Tox21_500537
66. Bdbm50132295
67. S2045
68. Capecitabine Impurity B (usp)
69. Akos015852921
70. Akos015896821
71. Tox21_111231_1
72. Ccg-204627
73. Cs-1270
74. Db12947
75. Ks-5065
76. Lp00537
77. Nsc 758890
78. Sdccgsbi-0050520.p002
79. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoropyrimidine-2,4-dione
80. Ncgc00093926-01
81. Ncgc00093926-02
82. Ncgc00093926-05
83. Ncgc00093926-11
84. Ncgc00261222-01
85. Ro-21-9738
86. Sri-11552-08
87. Sri-11552_09
88. 5''-deoxy-5-fluorouridine (5''-dfur)
89. Eu-0100537
90. D01309
91. F 8791
92. Capecitabine Related Compound B [usp-rs]
93. 094f095
94. Q1253473
95. Sr-01000075886-1
96. Sr-01000075886-5
97. 5'-dfur (5'-deoxy-5-fluorouridine, Doxifluridine)
98. Brd-k58262659-001-09-7
99. Capecitabine Related Compound B [usp Impurity]
100. 1-(.beta.-d-5-desoxyribofuranoxyl)-5-fluoruracil
101. Capecitabine Impurity B, European Pharmacopoeia (ep) Reference Standard
102. Capecitabine Related Compound B, United States Pharmacopeia (usp) Reference Standard
103. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-methyl-tetrahydrofuran-2-yl)-5-fluoropyrimidine-2,4(1h,3h)-dione
104. 1-((2r,5r)-3,4-dihydroxy-5-methyl-tetrahydro-furan-2-yl)-5-fluoro-1h-pyrimidine-2,4-dione
105. 1-((4s,2r,3r,5r)-3,4-dihydroxy-5-methyloxolan-2-yl)-5-fluoro-1,3-dihydropyrimidine-2,4-dione
106. 1-(5-deoxy-beta-d-ribofuranosyl)-5-fluoropyrimidine-2,4(1h,3h)-dione (5'-deoxy-5-fluorouridine)
107. Furtulon, Flutron, Doxyfluridine, Doxifluridina, Doxifluridinum 5'-doxifluridine, 5'dfurd, 5-fluoro-5'-deoxyuridine
| Molecular Weight | 246.19 g/mol |
|---|---|
| Molecular Formula | C9H11FN2O5 |
| XLogP3 | -1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 246.06519962 g/mol |
| Monoisotopic Mass | 246.06519962 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 399 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Appetite Stimulants
Agents that are used to stimulate appetite. These drugs are frequently used to treat anorexia associated with cancer and AIDS. (See all compounds classified as Appetite Stimulants.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)