
1. Adriablastin
2. Adriablastine
3. Adriamycin
4. Adriblastin
5. Adriblastina
6. Adriblastine
7. Adrimedac
8. Doxo Cell
9. Doxo-cell
10. Doxolem
11. Doxorubicin
12. Doxorubicin Hexal
13. Doxorubicin Nc
14. Doxorubicina Ferrer Farm
15. Doxorubicina Funk
16. Doxorubicina Tedec
17. Doxorubicine Baxter
18. Doxotec
19. Farmiblastina
20. Hydrochloride, Doxorubicin
21. Myocet
22. Onkodox
23. Ribodoxo
24. Rubex
25. Urokit Doxo Cell
26. Urokit Doxo-cell
1. 25316-40-9
2. Doxorubicin Hcl
3. Rubex
4. Adriacin
5. Adriblastina
6. Caelyx
7. Doxil
8. Adriamycin Hydrochloride
9. Doxorubicin (adriamycin) Hcl
10. Adriamycin, Hydrochloride
11. Doxorubicin (hydrochloride)
12. Hydroxydaunorubicin Hydrochloride
13. Adriablastina Cs
14. Adriblastin
15. Nsc 123127
16. Hydroxydaunomycin Hydrochloride
17. Mls001401460
18. 82f2g7bl4e
19. Adm Hydrochloride
20. Lipodox
21. Smr000058570
22. Adriamycin Pfs
23. Adriamycin Rdf
24. Dsstox_cid_10636
25. Dsstox_rid_78854
26. Dsstox_rid_80678
27. Dsstox_gsid_30636
28. Dsstox_gsid_45111
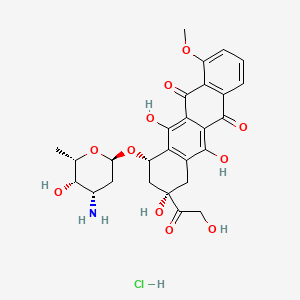
29. (8s,10s)-10-(((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Hydrochloride
30. (8s,10s)-10-{[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione Hydrochloride
31. Adrosal
32. Duxocin
33. Doxorubicin Citric Acid Salt
34. Adriamycin Hydrochloride-dna Complex
35. Mls000028393
36. Mfcd00077757
37. Lipo-dox
38. Fi 6804
39. (7s,9s)-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride
40. (8s-cis)-10-[(3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxynaphthacene-5,12-dione Hydrochloride
41. Nsc-123127
42. Sr-01000003049
43. Kw-125
44. Unii-82f2g7bl4e
45. Resmycin
46. Ccris 740
47. Doxorubicin.hcl
48. Doxorubicin Hydrochloride [jan]
49. Doxorubicin, Hcl
50. Adriamycin (tn)
51. Adriacin (tn)
52. (1s,3s)-3-glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-l-lyxo-hexopyranoside Hydrochloride
53. (8s,10s)-10-((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yloxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Hydrochloride
54. (8s-cis)-10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxynaphthacene-5,12-dione Hydrochloride
55. Prestwick_188
56. Einecs 246-818-3
57. Doxorubicin Hydrochloride [usp:jan]
58. Doxil (tn)
59. Rubex (tn)
60. Cpd000058570
61. Adriblastina Hydrochloride
62. (8s,10s)-10-(((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracen
63. Dox
64. Adriamycin [iarc]
65. Doxorubicini Hydrochloridum
66. Ncgc00163543-01
67. Schembl3242
68. Cas-25316-40-9
69. Mls000049969
70. Mls000049970
71. Mls000049971
72. Mls000070047
73. Mls000392861
74. Mls000392871
75. Mls000392881
76. Mls000392891
77. Mls000392901
78. Mls000759533
79. Mls001055359
80. Doxorubicin Hydrochloride,(s)
81. Chembl359744
82. Dtxsid3030636
83. Chebi:31522
84. Doxorubicin Hydrochloride- Bio-x
85. Doxorubicin Hydrochloride Solution
86. Liposomal Doxorubicin Hydrochloride
87. Bcpp000429
88. Hms1569g18
89. Kuc110341c
90. Ex-a1337
91. Tox21_110050
92. Tox21_112061
93. Tox21_202483
94. Adriamycin (doxorubicin Hydrochloride
95. S1208
96. Akos007930231
97. Akos015920241
98. Doxorubicin Hydrochloride (jp17/usp)
99. Tox21_110050_1
100. Bcp9000237
101. Ccg-100975
102. Cs-1239
103. Nc00225
104. 2-chloro-4-fluorobenzenesulphonylchloride
105. Doxorubicin Hydrochloride [mart.]
106. Doxorubicin Hydrochloride [vandf]
107. Doxorubicin Hydrochloride Liposome
108. Ncgc00014486-01
109. Ncgc00024415-33
110. Ncgc00024415-36
111. Ncgc00260032-01
112. Ncgc00263918-03
113. (8s,10s)-10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione Hydrochloride
114. As-31687
115. Bd164391
116. Doxorubicin Hydrochloride [usp-rs]
117. Doxorubicin Hydrochloride [who-dd]
118. Doxorubicin Hydrochloride [who-ip]
119. Hy-15142
120. Ksc-230-184-1
121. Doxorubicin Hydrochloride [ema Epar]
122. D01275
123. Doxorubicin Hydrochloride [ep Monograph]
124. Doxorubicin Hydrochloride [orange Book]
125. Doxorubicin Hydrochloride [usp Monograph]
126. 316d409
127. A817779
128. Doxorubicin Hydrochloride Liposome [vandf]
129. Doxorubicin Liposomal Complex Of The Hydrochloride
130. Doxorubicini Hydrochloridum [who-ip Latin]
131. Doxorubicin Hydrochloride, 98.0-102.0% (hplc)
132. Imx-110 Component Doxorubicin Hydrochloride
133. Liposomal Doxorubicin Hydrochloride [who-dd]
134. Sr-01000003049-5
135. Q27032359
136. Sr-01000003049-10
137. Z1557399790
138. Doxorubicin Liposomal Complex Of The Hydrochloride [mi]
139. Doxorubicin Hydrochloride, European Pharmacopoeia (ep) Reference Standard
140. Doxorubicin Hydrochloride, Suitable For Fluorescence, 98.0-102.0% (hplc)
141. Doxorubicin Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
142. Doxorubicin Hydrochloride, United States Pharmacopeia (usp) Reference Standard
143. (8s,10s)-10-(((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dionehydrochloride
144. (8s,10s)-10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione Hydrochloride
145. 10-[(3-amino-2,3,6-trideoxy-?-l-lyxohexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-5,12-naphthacenedione Hydrochloride
146. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl)-1-methoxy-, Hydrochloride (8s-cis)-
147. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, Hydrochloride, (8s,10s)-
148. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, Hydrochloride, (8s-cis)-
149. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl)-1-methoxy-, Hydrochloride (8s-cis)-
| Molecular Weight | 580.0 g/mol |
|---|---|
| Molecular Formula | C27H30ClNO11 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 5 |
| Exact Mass | 579.1507385 g/mol |
| Monoisotopic Mass | 579.1507385 g/mol |
| Topological Polar Surface Area | 206 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 977 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Doxorubicin hydrochloride |
| PubMed Health | Doxorubicin Hydrochloride Liposome (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Doxorubicin is a cytotoxic anthracycline antibiotic isolated from cultures of Streptomyces peucetius var. caesius. Doxorubicin consists of a naphthacenequinone nucleus linked through a glycosidic bond at ring atom 7 to an amino sugar, daunosamine. Ch... |
| Active Ingredient | Doxorubicin hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 10mg/vial; 200mg/100ml; 50mg/vial; 20mg/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Pharmachemie Bv; Onco Therapies; Sun Pharm Inds; Teva Pharms Usa; Sagent Pharms; Eurohlth Intl; Alvogen; Sandoz; Actavis |
| 2 of 2 | |
|---|---|
| Drug Name | Doxorubicin hydrochloride |
| PubMed Health | Doxorubicin Hydrochloride Liposome (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Doxorubicin is a cytotoxic anthracycline antibiotic isolated from cultures of Streptomyces peucetius var. caesius. Doxorubicin consists of a naphthacenequinone nucleus linked through a glycosidic bond at ring atom 7 to an amino sugar, daunosamine. Ch... |
| Active Ingredient | Doxorubicin hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 10mg/vial; 200mg/100ml; 50mg/vial; 20mg/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Pharmachemie Bv; Onco Therapies; Sun Pharm Inds; Teva Pharms Usa; Sagent Pharms; Eurohlth Intl; Alvogen; Sandoz; Actavis |
Myocet liposomal, in combination with cyclophosphamide, is indicated for the first-line treatment of metastatic breast cancer in adult women.
Caelyx pegylated liposomal is indicated:
- as monotherapy for patients with metastatic breast cancer , where there is an increased cardiac risk;
- for treatment of advanced ovarian cancer in women who have failed a first-line platinum-based chemotherapy regimen;
- in combination with bortezomib for the treatment of progressive multiple myeloma in patients who have received at least one prior therapy and who have already undergone or are unsuitable for bone marrow transplant;
- for treatment of AIDS-related Kaposis sarcoma (KS) in patients with low CD4 counts (< 200 CD4 lymphocytes/mm3) and extensive mucocutaneous or visceral disease.
Caelyx pegylated liposomal may be used as first-line systemic chemotherapy, or as second line chemotherapy in AIDS-KS patients with disease that has progressed with, or in patients intolerant to, prior combination systemic chemotherapy comprising at least two of the following agents: a vinca alkaloid, bleomycin and standarddoxorubicin (or other anthracycline).
Treatment of breast and ovarian cancer .
Treatment of hepatocellular carcinoma
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
L01DB01
L01DB
L01DB01