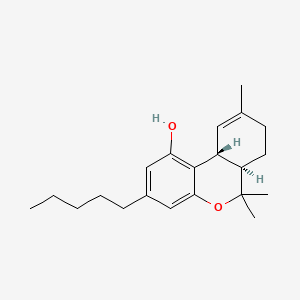

1. 9 Ene Tetrahydrocannabinol
2. 9-ene-tetrahydrocannabinol
3. Delta(1)-tetrahydrocannabinol
4. Delta(1)-thc
5. Delta(9)-tetrahydrocannabinol
6. Delta(9)-thc
7. Marinol
8. Tetrahydrocannabinol
9. Tetrahydrocannabinol, (6a-trans)-isomer
10. Tetrahydrocannabinol, (6ar-cis)-isomer
11. Tetrahydrocannabinol, (6as-cis)-isomer
12. Tetrahydrocannabinol, Trans Isomer
13. Tetrahydrocannabinol, Trans-(+-)-isomer
14. Tetrahydrocannabinol, Trans-isomer
15. Thc
1. Tetrahydrocannabinol
2. Marinol
3. Delta9-tetrahydrocannabinol
4. Delta9-thc
5. Delta-9-tetrahydrocannabinol
6. 1972-08-3
7. Abbott 40566
8. Deltanyne
9. Delta-9-thc
10. Delta(9)-thc
11. Thc
12. Dronabinolum
13. Delta(1)-tetrahydrocannabinol
14. Delta(9)-tetrahydrocannabinol
15. Delta1-thc
16. 1-trans-delta-9-tetrahydrocannabinol
17. Delta(sup 1)-thc
18. Delta(sup 9)-thc
19. Qcd 84924
20. Sp 104
21. Namisol
22. (-)-delta9-trans-tetrahydrocannabinol
23. 9-tetrahydrocannabinol
24. 1-trans-delta9-tetrahydrocannabinol
25. Qcd-84924
26. Delta1-tetrahydrocannabinol
27. Delta(9)-tetrahydrocannibinol
28. .delta.9-tetrahydrocannabinol
29. Syndros
30. Delta(sup 1)-tetrahydrocannabinol
31. Delta(sup 9)-tetrahydrocannabinol
32. .delta.1-thc
33. .delta.9-thc
34. L-delta1-trans-tetrahydrocannabinol
35. Cannabinol, Delta1-tetrahydro-
36. Ccris 4726
37. (6ar,10ar)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol
38. Chebi:66964
39. 3-pentyl-6,6,9-trimethyl-6a,7,8,10a-tetrahydro-6h-dibenzo(b,d)pyran-1-ol
40. Chembl465
41. Tetrahydrocannabinols (-)-delta1-3,4-trans-form
42. Nsc-134454
43. (6ar,10ar)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol
44. .delta.-9-tetrahydrocannabinol
45. Abbott-40566
46. 6465-30-1
47. Cannabinol, 1-trans-delta(sup 9)-tetrahydro-
48. J882f
49. 6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol
50. Sp-104
51. 6h-dibenzo(b,d)pyran-1-ol, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6ar-trans)-
52. 7j8897w37s
53. Dronabinolum [latin]
54. Tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo(b,d)pyran-1-ol
55. 6,6,9-trimethyl-3-pentyl-7,8,9,10-tetrahydro-6h-dibenzo(b,d)pyran-1-ol
56. Tetrahydrocannabinols (-)-trans-.delta.9-form
57. (6ar,10ar)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo(b,d)pyran-1-ol
58. 6h-dibenzo(b,d)pyran-1-ol, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6ar,10ar)-
59. (-)-.delta.9-thc
60. Compassia
61. Relivar
62. (l)-.delta.1-tetrahydrocannabinol
63. (-)-.delta.1-tetrahydrocannabinol
64. (-)-.delta.9-tetrahydrocannabinol
65. L-.delta.1-trans-tetrahydrocannabinol
66. L-trans-.delta.9-tetrahydrocannabinol
67. (6ar-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo(b,d)pyran-1-ol
68. 6h-dibenzo[b,d]pyran-1-ol, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6ar,10ar)-
69. (-)-.delta.9-trans-tetrahydrocannabinol
70. (-)-trans-.delta.9-tetrahydrocannabinol
71. Marinol (tn)
72. Dea No. 7369
73. Delta1-tetrahydrocannabinol (van)
74. Delta9-tetrahydrocannabinol (van)
75. Dronabinol (usp/inn)
76. .delta.1-tetrahydrocannabinol
77. Tetrahydrocannabinol Delta9
78. (-)-trans-delta9-thc
79. (l)-delta(sup 1)-tetrahydrocannabinol
80. Cannabinol, .delta.1-tetrahydro-
81. 1-trans-delta(sup 9)-tetrahydrocannabinol
82. .delta.9-trans-tetrahydrocannabinol
83. (l)-delta1-tetrahydrocannabinol
84. L-.delta.1-tetrahydrocannabinol
85. (-)-delta1-tetrahydrocannabinol
86. (-)-delta9-tetrahydrocannabinol
87. Delta9-trans-tetrahydrocannabinol
88. Hsdb 6471
89. Trans-delta9-tetrahydrocannabinol
90. (6ar,10ar)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo[b,d]pyran-1-ol
91. (6ar-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo[b,d]pyran-1-ol
92. Cat-310
93. 6h-dibenzo(b,d)pyran-1-ol, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, Trans-
94. L-trans-delta9-tetrahydrocannabinol
95. (-)-delta(sup 1)-3,4-trans-tetrahydrocannabinol
96. 6h-dibenzo[b,d]pyran-1-ol, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6ar-trans)-
97. Drg-0138
98. Tci
99. 14c-.delta.1-tetrahydrocannabinol
100. Trans-.delta.9-tetrahydrocannabinol
101. (-)-trans-delta1-tetrahydrocannabinol
102. (-)-trans-delta9-tetrahydrocannabinol
103. Nsc 134454
104. .delta.-9-thc
105. 1-trans-delta(sup9)-tetrahydrocannabinol
106. Dronabinol (synthetic)
107. .delta.(sup9)-thc
108. 3ls4
109. Dronabinol [inn]
110. (-)-3,4-trans-delta1-tetrahydrocannabinol
111. 1-trans-.delta.(sup9)-tetrahydrocannabinol
112. Dronabinol [hsdb]
113. Dronabinol [usan]
114. Dsstox_cid_1327
115. (-)-.delta.(sup9)-trans-tetrahydrocannabinol
116. Dronabinol [vandf]
117. Epitope Id:224552
118. Dronabinol [mart.]
119. Schembl4609
120. Delta 9-tetrahydrocannabinol
121. Delta-9 Tetrahydrocannabinol
122. Dronabinol [who-dd]
123. Dsstox_rid_76083
124. Trans-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6h-dibenzo(b,d)pyran-1-ol
125. Dsstox_gsid_21327
126. Bidd:gt0427
127. Dronabinol [usan:usp:inn]
128. Gtpl2424
129. Cannabinol, Tetrahydro- (6ci)
130. Dronabinol [orange Book]
131. Dtxsid6021327
132. Unii-7j8897w37s
133. Bdbm60994
134. Dronabinol [usp Monograph]
135. Dtxsid001038830
136. Us9416103, Delta9-thc
137. (+/-)-delta9-tetrahydrocannabinol
138. Int-0010
139. Zinc1530625
140. Tox21_112616
141. Bdbm50007391
142. Nsc134454
143. Pdsp2_000714
144. Cannabinol, Delta1-tetrahydro- (7ci)
145. Db00470
146. Int-0010/06
147. (-)-delta9-tetrahydrocannabinol Solution
148. Cas-1972-08-3
149. Tetrahydrocannabinol, Delta-9 Trans
150. Synthetic Thc In Sesame Oil / Soft Gelatin
151. C06972
152. Cannabinol, 1-trans-.delta.(sup9)-tetrahydro-
153. D00306
154. Dronabinol In Sesame Oil In Soft Gelatin Capsule
155. Q190067
156. (-)-.delta.1-3,4-trans-tetrahydrocannabinol
157. (-)-delta9-thc (dronabinol) 0.1 Mg/ml In Methanol
158. (-)-delta9-thc (dronabinol) 1.0 Mg/ml In Methanol
159. (-)-delta9-thc (dronabinol) 5.0 Mg/ml In Methanol
160. Delta9-tetrahydrocannabinol Solution, Ethanol Solution
161. Delta9-tetrahydrocannabinol 250 Microg/ml In Acetonitrile
162. Tetrahydrocannabinols (-)-trans-.delta.9-form [mi]
163. Delta-9-tetrahydrocannabinol (cannabis Sativa Extract)
164. 6h-dibenzo[b, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-
165. (-)-delta9-tetrahydrocannabinol (delta9-thc) 100 Microg/ml In Methanol
166. (-)-delta9-tetrahydrocannabinol (delta9-thc) 1000 Microg/ml In Methanol
167. 6,9-trimethyl-3-pentyl-7,8,9,10-tetrahydro-6h-dibenzo[b,d]pyran-1-ol
168. 6h-dibenzo[b, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, Trans-
169. (-)-(6ar,10ar)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol
170. (-)-delta(sup 1)-3,4-trans-tetrahydrocannabinol(l)-delta(sup 1)-tetrahydrocannabinol
171. (10r,10ar)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol
172. (6ar,10ar)-6,6,9-trimethyl-3-pentyl-6h,6ah,7h,8h,10ah-benzo[c]isochromen-1-ol
173. (s)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol
174. 6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol(deltae-9-thc)
175. 6h-dibenzo[b, 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6ar-trans)-
176. Delta-9-tetrahydrocannabinol, United States Pharmacopeia (usp) Reference Standard
177. (-)-delta9-tetrahydrocannabinol Solution, ~1 Mg/ml In Ethanol, Analytical Standard, For Drug Analysis
178. (-)-trans-delta9-thc Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
179. (6ar,10ar)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-dibenzo(b,d)pyran-1-ol
180. 6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6h-benzo[c]chromen-1-ol(delta9-thc(delta9-tetrahydrocannabinol))
181. 8,8-dimethyl-11-methylene-5-pentyl-3,4,8a,9,10,11,12,12a-octahydro-2h,8h-1,7-dioxa-benzo[c]phenanthrene
182. Delta9-tetrahydrocannabinol Solution, 1.0 Mg/ml In Methanol, Analytical Standard, For Drug Analysis
| Molecular Weight | 314.5 g/mol |
|---|---|
| Molecular Formula | C21H30O2 |
| XLogP3 | 7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 314.224580195 g/mol |
| Monoisotopic Mass | 314.224580195 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 439 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Dronabinol |
| PubMed Health | Dronabinol (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in MARINOL... |
| Active Ingredient | Dronabinol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Svc Pharma; Insys Therap; Akorn |
| 2 of 4 | |
|---|---|
| Drug Name | Marinol |
| PubMed Health | Dronabinol (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in MARINOL... |
| Active Ingredient | Dronabinol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Abbvie |
| 3 of 4 | |
|---|---|
| Drug Name | Dronabinol |
| PubMed Health | Dronabinol (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in MARINOL... |
| Active Ingredient | Dronabinol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Svc Pharma; Insys Therap; Akorn |
| 4 of 4 | |
|---|---|
| Drug Name | Marinol |
| PubMed Health | Dronabinol (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in MARINOL... |
| Active Ingredient | Dronabinol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Abbvie |
Hallucinogens Psychotropic Drugs Analgesics, Non-Narcotic Cannabinoid Receptor Agonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2017); Available from, as of September 13, 2017: https://meshb.nlm.nih.gov/record/ui?ui=D013759
Dronabinol capsules are indicated for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
Dronabinol is used for the treatment of anorexia associated with weight loss in patients with acquired immunodeficiency syndrome (AIDS). Dronabinol is designated an orphan drug by the US Food and Drug Administration (FDA) for use in this condition.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011
/EXPL THER/ BACKGROUND: Cannabis use disorder is associated with substantial morbidity and, after alcohol, is the most common drug bringing adolescents and adults into treatment. At present, there are no FDA-approved medications for cannabis use disorder. Combined pharmacologic interventions might be particularly useful in mitigating withdrawal symptoms and promoting abstinence. OBJECTIVE: The purpose of this study was to evaluate the safety and efficacy of dronabinol, a synthetic form of delta-9-tetrahydrocannabinol, a naturally occurring pharmacologically active component of marijuana, and lofexidine, an alpha-2 agonist, in treating cannabis dependence. METHODS: One hundred fifty six cannabis-dependent adults were enrolled and following a 1-week placebo lead-in phase 122 were randomized in a double-blind, placebo-controlled, 11-week trial. Participants were randomized to receive dronabinol 20 mg three times a day and lofexidine 0.6 mg three times a day or placebo. Medications were maintained until the end of week eight, were then tapered over two weeks and patients were monitored off medications during the last study week. All participants received weekly motivational enhancement and relapse prevention therapy. Marijuana use was assessed using the timeline follow-back method. RESULTS: There was no significant difference between treatment groups in the proportion of participants who achieved 3 weeks of abstinence during the maintenance phase of the trial (27.9% for the medication group and 29.5% for the placebo group), although both groups showed a reduction over time. CONCLUSIONS: Based on this treatment study, the combined intervention did not show promise as a treatment for cannabis use disorder.
PMID:26711160 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4729291 Levin FR et al; Drug Alcohol Depend 159: 53-60 (2016)
For more Therapeutic Uses (Complete) data for delta 9-Tetrahydrocannabinol (10 total), please visit the HSDB record page.
Dronabinol capsules are contraindicated in any patient who has a known sensitivity to dronabinol or any of its ingredients. It contains cannabinoid and sesame oil and should never be used by patients allergic to these substances.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
Patients receiving treatment with dronabinol capsules should be specifically warned not to drive, operate machinery, or engage in any hazardous activity until it is established that they are able to tolerate the drug and to perform such tasks safely.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
Seizure and seizure-like activity have been reported in patients receiving dronabinol capsules during marketed use of the drug and in clinical trials. Dronabinol capsules should be used with caution in patients with a history of seizure disorder because dronabinol capsules may lower the seizure threshold. A causal relationship between dronabinol capsules and these events has not been established. Dronabinol capsules should be discontinued immediately in patients who develop seizures and medical attention should be sought immediately.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
Dronabinol capsules should be used with caution in patients with cardiac disorders because of occasional hypotension, possible hypertension, syncope, or tachycardia.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
For more Drug Warnings (Complete) data for delta 9-Tetrahydrocannabinol (15 total), please visit the HSDB record page.
The estimated lethal human dose of intravenous dronabinol is 30 mg/kg (2100 mg/70 kg).
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
For the treatment of anorexia associated with weight loss in patients with AIDS, and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments
FDA Label
Treatment of central and peripheral neuropathic pain
Treatment of spasticity
Marinol may has complex effects on the central nervous system (CNS), including cannabinoid receptors. Dronabinol may inhibit endorphins in the emetic center, suppress prostaglandin synthesis, and/or inhibit medullary activity through an unspecified cortical action.
Cannabinoid Receptor Agonists
Compounds that interact with and stimulate the activity of CANNABINOID RECEPTORS. (See all compounds classified as Cannabinoid Receptor Agonists.)
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Psychotropic Drugs
A loosely defined grouping of drugs that have effects on psychological function. Here the psychotropic agents include the antidepressive agents, hallucinogens, and tranquilizing agents (including the antipsychotics and anti-anxiety agents). (See all compounds classified as Psychotropic Drugs.)
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AD - Other antiemetics
A04AD10 - Dronabinol
Absorption
Dronabinol capsules are almost completely absorbed (90 to 95%) after single oral doses. Due to the combined effects of first pass hepatic metabolism and high lipid solubility, only 10 to 20% of the administered dose reaches the systemic circulation. After oral administration, dronabinol has an onset of action of approximately 0.5 to 1 hours and peak effect at 2 to 4 hours. Following BID dosing of 2.5mg of dronabinol, Cmax was found to be 1.32ng/mL with a median Tmax of 1.00 hr.
Route of Elimination
Dronabinol and its biotransformation products are excreted in both feces and urine. Because of its large volume of distribution, dronabinol and its metabolites may be excreted at low levels for prolonged periods of time. Following single dose administration, low levels of dronabinol metabolites have been detected for more than 5 weeks in the urine and feces.
Volume of Distribution
Dronabinol has a large apparent volume of distribution, approximately 10 L/kg, because of its lipid solubility.
Clearance
Values for clearance average about 0.2 L/kg-hr, but are highly variable due to the complexity of cannabinoid distribution.
To establish a fast sensitive, reproducible LC-MS/MS method to study pharmacokinetic properties of THC, and compare relative bioavailability of THC and its solid dispersion in mice. 200 mice were divided randomly into two groups, and administered orally with THC and THC-solid dispersion after fasting (calculate on THC:400 mg x kg(-1)), used HPLC-MS/MS method to determine the THC concentration of each period at the following times: baseline ( predose ), 15, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 24 hr after dosing. Calculating the pharmacokinetic parameters according to the C-t curv, and then use the Phoenix WinNonlin software for data analysis. The calibration curves were linear over the range 9.06-972 ug/L for THC (R2 = 0.999). The limit of detection (LOD) was 0.7 ug/L, respectively. The average extraction recoveries for THC was above 75%, The methodology recoveries were between 79% and 108%. The intra-day and inter-day RSD were less than 13%, the stability test showed that the plasma samples was stable under different conditions (RSD < 15%). The precision, accuracy, recovery and applicability were found to be adequate for pharmacokinetic studies. Pharmacokinetic parameters of THC and THC-solid dispersion orally to mice shows as fllows: T(max), were 60 and 15 min, AUC(0-t) were 44 00.43 and 57 497.81 mg x L(-1) x min, AUC(0-infinity) were 51226.00 and 68031.48 mg/L x min, MRT(0-infinity) were 596.915 6, 661.747 7 min, CL(z)/F were 0.007 809 and 0.005 88 L/min x kg. Compared with THC, the MRT and t1/2 of the THC-solid dispersion were all slightly extended, the t(max) was significantly reduced, AUC(0-24 hr), AUC(0-infinity) and C(max) were all significantly higher, the relative bioavailability of THC-solid dispersion is 1.34 times of THC. The results of the experiment shows that the precision, accuracy, recovery and applicability were found to be adequate for the pharmacokinetic studies. After oral administration to mice, the relative bioavailability of THC-solid dispersion show significant improvement compared to THC.
PMID:24956859 Liao L et al; Zhongguo Zhong Yao Za Zhi 39 (6): 1101-6 (2014)
/MILK/ Marijuana has not been well studied, but tetrahydrocannabinol can reach rather high levels in /breast/ milk, particularly with heavy use. Although adverse effects in infants have not been reported, breast feeding should probably be avoided in heavy users and during therapeutic dronabinol use. Breast feeding should probably be withheld for several hr after occasional marijuana use. Caution should also be used to avoid exposing the infant to marijuana smoke.
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 178
There is a great concern about the safety of THC-based drugs in older people (=65 years), as most of THC-trials did not include such group. In this phase 1, randomized, double-blind, double-dummy, placebo-controlled, cross-over trial, we evaluated the safety and pharmacokinetics of three oral doses of Namisol(), a novel THC in tablet form, in older subjects. Twelve healthy older subjects (6 male; mean age 72+/-5 years) randomly received a single oral dose of 3 mg, 5 mg, or 6.5 mg of THC or matching placebo, in a crossover manner, on each intervention day. The data for 11 subjects were included in the analysis. The data of 1 subject were excluded due to non-compliance to study medication. THC was safe and well tolerated. The most frequently reported adverse events (AEs) were drowsiness (27%) and dry mouth (11%). Subjects reported more AEs with THC 6.5mg than with 3mg (p=0.048), 5mg (p=0.034) and placebo (p=0.013). There was a wide inter-individual variability in plasma concentrations of THC. Subjects for whom the Cmax fell within the sampling period (over 2hr), Cmax was 1.42-4.57 ng/mL and Tmax was 67-92 min. The AUC0-2 hr (n=11) was 1.67-3.51 ng/mL. Overall, the pharmacodynamic effects of THC were smaller than effects previously reported in young adults. In conclusion, THC appeared to be safe and well tolerated by healthy older individuals. Data on safety and effectiveness of THC in frail older persons are urgently required, as this population could benefit from the therapeutic applications of THC.
PMID:25035121 Ahmed AI et al; Eur Neuropsychopharmacol 24 (9): 1475-82 (2014)
RATIONALE: Data on safety, pharmacodynamics, and pharmacokinetics of tetrahydrocannabinol (THC) are lacking in dementia patients. METHODS: In this randomized, double-blind, placebo-controlled, crossover trial, we evaluated the safety, pharmacodynamics, and pharmacokinetics of THC in ten patients with dementia (mean age 77.3 +/-5.6). For 12 weeks, participants randomly received oral THC (weeks 1-6, 0.75 mg; weeks 7-12, 1.5 mg) or placebo twice daily for 3 days, separated by a 4-day washout period. RESULTS: Only 6 of the 98 reported adverse events were related to THC. Visual analog scale (VAS) feeling high, VAS external perception, body sway-eyes-open, and diastolic blood pressure were not significantly different with THC. After the 0.75-mg dose, VAS internal perception (0.025 units; 95% CI 0.010-0.040) and heart rate (2 beats/min; 95% CI 0.4-3.8) increased significantly. Body sway-eyes-closed increased only after 1.5 mg (0.59 deg/s; 95% CI 0.13-1.06). Systolic blood pressure changed significantly after both doses of THC (0.75 mg, -7 mmHg, 95% CI -11.4, -3.0; 1.5 mg, 5 mmHg, 95% CI 1.0-9.2). The median T max was 1-2 hr, with THC pharmacokinetics increasing linearly with increasing dose, with wide interindividual variability (CV% up to 140%). The mean C max (ng/mL) after the first dose (0-6 hr) was 0.41 (0.18-0.90) for the 0.75-mg dose and 1.01 (0.53-1.92) for the 1.5-mg dose. After the second dose (6-24 hr), the C max was 0.50 (0.27-0.92) and 0.98 (0.46-2.06), respectively. CONCLUSIONS: THC was rapidly absorbed and had dose-linear pharmacokinetics with considerable interindividual variation. Pharmacodynamic effects, including adverse events, were minor. Further studies are warranted to evaluate the pharmacodynamics and efficacy of higher THC doses in older persons with dementia.
PMID:25752889 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4480847 Ahmed AI et al; Psychopharmacology (Berl) 232 (14): 2587-95 (2015)
For more Absorption, Distribution and Excretion (Complete) data for delta 9-Tetrahydrocannabinol (32 total), please visit the HSDB record page.
THC is primarily metabolized in the liver by microsomal hydroxylation and oxidation reactions catalyzed by Cytochrome P450 enzymes. 11-hydroxy-9-tetrahydrocannabinol (11-OH-THC) is the primary active metabolite, capable of producing psychological and behavioural effects, which is then metabolized into 11-nor-9-carboxy- 9-tetrahydrocannabinol (THC-COOH), THC's primary inactive metabolite. Dronabinol and its principal active metabolite, 11-OH-delta-9-THC, are present in approximately equal concentrations in plasma. Concentrations of both parent drug and metabolite peak at approximately 0.5 to 4 hours after oral dosing and decline over several days.
The analgesic activity of delta-8- and delta-9-THC is mainly due to the 11-hydroxy metabolite.
Wilson RS, May EL; J Med Chem 18: 700-3 (1975) as cited in DHHS/NIDA; Research Monograph Series 52: Testing Drugs for Physical Dependence Potential and Abuse Liability p.71 (1984) DHHS Pub No. (ADM)87-1332
The metabolism of delta(9)-tetrahydrocannabinol (THC) is relatively complex, and over 80 metabolites have been identified. However, much less is known about the formation and fate of cannabinoid conjugates. Bile excretion is known to be an important route for the elimination of phase II metabolites. A liquid chromatography-tandem mass spectrometry LC-MS/MS procedure for measuring cannabinoids in oral fluid was adapted, validated and applied to 10 bile samples. THC, 11-hydroxy-delta(9)-tetrahydrocannabinol (11-OH-THC), 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol (THCCOOH), cannabinol (CBN), cannabidiol (CBD), delta(9)-tetrahydrocannabinolic acid A (THC-A), 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol glucuronide (THCCOOH-gluc) and delta(9)-tetrahydrocannabinol glucuronide (THC-gluc) were determined following solid-phase extraction and LC-MS/MS. High concentrations of THCCOOH-gluc were found in bile samples (range: 139-21,275 ng/mL). Relatively high levels of THCCOOH (7.7-1548 ng/mL) and THC-gluc (38-1366 ng/mL) were also measured. THC-A, the plant precursor of THC, was the only cannabinoid that was not detected. These results show that biliary excretion is an important route of elimination for cannabinoids conjugates and that their enterohepatic recirculation is a significant factor to consider when analyzing blood elimination profiles of cannabinoids. Furthermore, we suggest that the bile is the matrix of choice for the screening of phase II cannabinoid metabolites.
PMID:22980143 Fabritius M et al; Forensic Sci Int 223 (1-3): 114-8 (2012)
The presence of the metabolite 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (C-THC) in hair is generally accepted as the definitive proof of delta-9-tetrahydrocannabinol (THC) ingestion. During hair analysis, the removal of any potential C-THC external contamination that could result from marijuana smoke or close personal contact via a wash procedure is critical. Here, we performed a series of experiments to demonstrate that C-THC is the reliable indicator of marijuana ingestion when paired with the correct washing procedure to remove potential external contamination.
PMID:27185816 Hill VA et al; J Anal Toxicol 40 (5): 345-9 (2016)
Dronabinol undergoes extensive first-pass hepatic metabolism, primarily by microsomal hydroxylation, yielding both active and inactive metabolites. Dronabinol and its principal active metabolite, 11-OH-delta-9-THC, are present in approximately equal concentrations in plasma.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
For more Metabolism/Metabolites (Complete) data for delta 9-Tetrahydrocannabinol (15 total), please visit the HSDB record page.
Delta-9-Tetrahydrocannabinol has known human metabolites that include 11-hydroxy-delata-Tetrahydrocannabinol, 7-hydroxy-delta-9-Tetrahydrocannabinol, 7alpha-hydroxy-delta-9-Tetrahydrocannabinol, 8-hydroxy-delta-9-Tetrahydrocannabinol, and 8alpha-hydroxy-delta-9-Tetrahydrocannabinol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination phase of dronabinol can be described using a two compartment model with an initial (alpha) half-life of about 4 hours and a terminal (beta) half-life of 25 to 36 hours.
The elimination phase of dronabinol can be described using a two compartment model with an initial (alpha) half-life of about 4 hours and a terminal (beta) half-life of 25 to 36 hours.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
After the pseudo-equilibrium is reached, THC is then slowly eliminated. This slow elimination is primarily due to the slow return of THC from sequestered tissues to blood. The terminal half-life of THC is approximately 1 day. /Several investigators have/ suggested terminal half-life /ranging between/ 19 to 36 hours. There are no significant differences in plasma profiles for chronic and infrequent users or in half-lives in moderate users before and after a 2-week exposure of THC.
DHHS/NIDA; Research Monograph Series 79: Structure-Activity Relationships of the Cannabinoids p.XX (1987) DHHS Pub No. (ADM)90-1534
The elimination half-life of dronabinol (delta-1-tetrahydrocannabinol) in 3 chronic marihuana users following smoking 4 cigarettes over a 2 day period is reported. The elimination half-life in blood plasma was calculated to be 4.1 days (range 2.9-5.0 days).
Johansson E et al; J Pharm Pharmcol 40: 374-5 (1988)
The terminal plasma half-life of metabolites from THC administration is approximately 50 hours, which is longer than that of THC. ... /Metabolites/
DHHS/NIDA; Research Monograph Series 79: Structure-Activity Relationships of the Cannabinoids p.180 (1987) DHHS Pub No. (ADM)90-1534
Dronabinol is a synthetic form of delta-9-tetrahydrocannabinol (-THC), the primary psychoactive component of cannabis (marijuana). THC demonstrates its effects through weak partial agonist activity at Cannabinoid-1 (CB1R) and Cannabinoid-2 (CB2R) receptors, which results in the well-known effects of smoking cannabis such as increased appetite, reduced pain, and changes in emotional and cognitive processes.
The availability of potent synthetic agonists for cannabinoid receptors has facilitated our understanding of cannabinoid actions on synaptic transmission in the central nervous system. Moreover, the ability of these compounds to inhibit neurotransmitter release at many central synapses is thought to underlie most of the behavioral effects of cannabinoid agonists. However, despite the widespread use and misuse of marijuana, and recognition of its potential adverse psychological effects in humans, comparatively few studies have examined the actions of its primary psychoactive constituent, delta(9)-tetrahydrocannabinol (THC), at well-defined synaptic pathways. Here we examine the recent literature describing the effects of acute and repeated THC exposure on synaptic function in several brain regions and explore the importance of these neurobiological actions of THC in drug addiction.
PMID:23209160 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3721267 Hoffman AF, Lupica CR; Cold Spring Harb Perspect Med 3 (8). pii: a012237 (2013)
Cannabis has potential therapeutic use but tetrahydrocannabinol (THC), its main psychoactive component, appears as a risk factor for ischemic stroke in young adults. We therefore evaluate the effects of THC on brain mitochondrial function and oxidative stress, key factors involved in stroke. Maximal oxidative capacities V max (complexes I, III, and IV activities), V succ (complexes II, III, and IV activities), V tmpd (complex IV activity), together with mitochondrial coupling (V max/V 0), were determined in control conditions and after exposure to THC in isolated mitochondria extracted from rat brain, using differential centrifugations. Oxidative stress was also assessed through hydrogen peroxide (H2O2) production, measured with Amplex Red. THC significantly decreased V max (-71%; P<0.0001), V succ (-65%; P<0.0001), and V tmpd (-3.5%; P<0.001). Mitochondrial coupling (V max/V 0) was also significantly decreased after THC exposure (1.8+/-0.2 versus 6.3+/-0.7; P<0.001). Furthermore, THC significantly enhanced H2O2 production by cerebral mitochondria (+171%; P<0.05) and mitochondrial free radical leak was increased from 0.01+/-0.01 to 0.10+/-0.01% (P<0.001). Thus, THC increases oxidative stress and induces cerebral mitochondrial dysfunction. This mechanism may be involved in young cannabis users who develop ischemic stroke since THC might increase patient's vulnerability to stroke.
PMID:25654095 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4310259 Wolff V et al; Biomed Res Int 2015: 323706 (2015)
MDSCs are potent immunosuppressive cells that are induced during inflammatory responses, as well as by cancers, to evade the antitumor immunity. We recently demonstrated that marijuana cannabinoids are potent inducers of MDSCs. In the current study, we investigated the epigenetic mechanisms through which THC, an exogenous cannabinoid, induces MDSCs and compared such MDSCs with the naive MDSCs found in BM of BL6 (WT) mice. Administration of THC into WT mice caused increased methylation at the promoter region of DNMT3a and DNMT3b in THC-induced MDSCs, which correlated with reduced expression of DNMT3a and DNMT3b. Furthermore, promoter region methylation was decreased at Arg1 and STAT3 in THC-induced MDSCs, and consequently, such MDSCs expressed higher levels of Arg1 and STAT3. In addition, THC-induced MDSCs secreted elevated levels of S100A8, a calcium-binding protein associated with accumulation of MDSCs in cancer models. Neutralization of S100A8 by use of anti-S100A8 (8H150) in vivo reduced the ability of THC to trigger MDSCs. Interestingly, the elevated S100A8 expression also promoted the suppressive function of MDSCs. Together, the current study demonstrates that THC mediates epigenetic changes to promote MDSC differentiation and function and that S100A8 plays a critical role in this process.
PMID:25713087 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4370051 Sido JM et al; J Leukoc Biol 97 (4): 677-88 (2015)
Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids.
US Natl Inst Health; DailyMed. Current Medication Information for DRONABINOL capsule (September 2015). Available from, as of September 13, 2017: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=68b4168b-5782-4e68-a25a-5b4e4408dbce
For more Mechanism of Action (Complete) data for delta 9-Tetrahydrocannabinol (10 total), please visit the HSDB record page.