

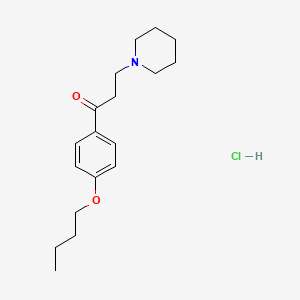

1. Dyclone
2. Dyclonine
3. Dyclonine Hcl
4. Sucrets
5. Tanac
1. 536-43-6
2. Dyclonine Hcl
3. Dyclone
4. Dyclothane
5. Dyclocaine Hydrochloride
6. Tanaclone
7. Dyclonine (hydrochloride)
8. Diclonina
9. 4'-butoxy-3-piperidinopropiophenone Hydrochloride
10. 1-propanone, 1-(4-butoxyphenyl)-3-(1-piperidinyl)-, Hydrochloride
11. P-267
12. Nsc-23018
13. 1-(4-butoxyphenyl)-3-piperidin-1-ylpropan-1-one;hydrochloride
14. Diclonia
15. Dyclonine Hydrochloride [usp]
16. Mls000069532
17. Zec193879q
18. Dyclocainum
19. Mfcd00035386
20. Smr000038382
21. Dsstox_cid_25323
22. Dsstox_rid_80802
23. Dsstox_gsid_45323
24. Dyclonine Hydrochloride (usp)
25. Wln: T6ntj A2vr Do4 &gh
26. Chebi:4725
27. Dycloninehydrochloride
28. Dyclonine, Hydrochloride
29. Ncgc00016498-01
30. Cas-536-43-6
31. Einecs 208-633-6
32. Nsc 23018
33. 4-n-butoxy-.beta.-(1-piperidyl)propiophenone Hydrochloride
34. Unii-zec193879q
35. Sr-01000000032
36. S 154
37. C 5422
38. Dyclopro (tn)
39. Prestwick_674
40. Dyclone (tn)
41. Piperidine, 1-(2-(4-butoxybenzoyl)ethyl), Hydrochloride
42. Opera_id_1265
43. Mls001077359
44. Mls002222240
45. Schembl317219
46. Spectrum1500268
47. Propiophenone, 4'-butoxy-3-piperidino-, Hydrochloride
48. Chembl1200478
49. Dtxsid6045323
50. Hy-b0364a
51. Hms1568f10
52. Hms1920k06
53. Pharmakon1600-01500268
54. Bcp28402
55. Nsc23018
56. Nsc25588
57. Dyclonine Hydrochloride [mi]
58. Tox21_110459
59. Ccg-40231
60. Nsc-25588
61. Nsc756745
62. S2041
63. Akos015951364
64. Tox21_110459_1
65. Ac-8346
66. Ds-3341
67. Dyclonine Hydrochloride [mart.]
68. Dyclonine Hydrochloride [vandf]
69. Nsc-756745
70. Dyclonine Hydrochloride [usp-rs]
71. Dyclonine Hydrochloride [who-dd]
72. Ncgc00016498-08
73. Ncgc00094662-01
74. Ncgc00094662-02
75. Ncgc00094662-03
76. Ncgc00094662-04
77. Sy066681
78. D4303
79. Dyclonine Hydrochloride [orange Book]
80. Ft-0625635
81. Sw196829-3
82. Dyclonine Hcl;dyclone;dyclocaine Hydrochloride
83. D00735
84. Dyclonine Hydrochloride [usp Monograph]
85. N12006
86. A829700
87. Sr-01000000032-4
88. W-105718
89. Q27106453
90. 4-butoxy-3-piperidinopropiophenone Hydrochloride
91. 4-n-butoxy-.beta.-piperidonopropiophenone Hydrochloride
92. 1-(4-butoxyphenyl)-3-(1-piperidinyl)-1-propanone Hydrochloride
93. 1-(4-butoxyphenyl)-3-piperidin-1-ylpropan-1-one;hydron;chloride
94. 1-propanone, 1-(4-butoxyphenyl)-3-(1-piperidinyl)hydrochloride
95. Dyclonine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
96. Dyclonine Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 325.9 g/mol |
|---|---|
| Molecular Formula | C18H28ClNO2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 325.1808568 g/mol |
| Monoisotopic Mass | 325.1808568 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 292 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)