

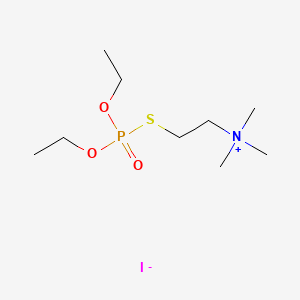

1. 2-((diethoxyphosphinyl)thio)-n,n,n,-trimethylethanaminium Iodide
2. Ecothiopate Iodide
3. Ecothiophate Iodide
4. Iodide, Echothiophate
5. Iodide, Ecothiopate
6. Iodide, Ecothiophate
7. Iodide, Phospholine
8. Phospholine Iodide
1. Ecothiopate Iodide
2. 513-10-0
3. Echodide
4. Ecothiophate Iodide
5. Phospholine Iodide
6. Ecothiopati Iodidum
7. Iodure D'ecothiopate
8. Ioduro De Ecotiopato
9. Diethoxyphosphoryl-thiocholine Iodide
10. 217-mi
11. Ecostigmine Iodide
12. Chebi:59849
13. Ethanaminium, 2-((diethoxyphosphinyl)thio)-n,n,n-trimethyl-, Iodide
14. 2-diethoxy-phosphinylthioethyl-trimethylammonium Iodide
15. N-(2-(diethoxyphosphinylthio)ethyl)trimethylammonium Iodide
16. Ecothiopate Iodide [inn]
17. O,o-diethyl S-2-trimethylammonium Ethylphosphonothiolate Iodide
18. Echothiophate Iodide (usp)
19. Echothiophate Iodide [usp]
20. Ba9qh3p00t
21. S-(2-(n,n,n-trimethylammonio)ethyl) O,o-diethylphosphorothiolate Iodide
22. (2-mercaptoethyl)trimethylammonium Iodide S-ester With O,o-diethyl Phosphorothioate
23. Ecostigmini Jodidum
24. 2-diethoxyphosphorylsulfanylethyl(trimethyl)azanium;iodide
25. Phospholine (the Pharmaceutical)
26. Ecothiopati Iodidum [inn-latin]
27. Iodure D'ecothiopate [inn-french]
28. Ioduro De Ecotiopato [inn-spanish]
29. 217 Mi
30. 217mi; Diethoxyphosphinylthiocholine Iodide; Diethylphosphorylthiocholine Iodide; Echothiopate Iodide; Ecostigmini Jodidum;
31. Phospholine Iodide (tn)
32. Einecs 208-152-1
33. Unii-ba9qh3p00t
34. Ethanaminium, 2-[(diethoxyphosphinyl)thio]-n,n,n-trimethyl-, Iodide
35. 2-diaethoxyphosphinyl-thioaethyl-trimethyl-ammonium-jodid [german]
36. Ammonium, (2-(o,o-diethylphosphorothio)ethyl)trimethyl-, Iodide
37. S-(2-dimethylaminoethyl)-o.o-diethylphosphorothioate Methiodide
38. Diethoxyphosphinylthiocholine Iodide
39. Echothiopate Iodide
40. S-ester Of (2-mercaptoethyl)trimethylammonium Iodide With O,o-diethyl Phosphorothioate
41. 2-diaethoxyphosphinyl-thioaethyl-trimethyl-ammonium-jodid
42. Ecothiopateiodide
43. S-beta-dimethylaminoethyl-o,o-diethylthionophosphate Methiodide
44. Ammonium, (2-mercaptoethyl)trimethyl-, Iodide, S-ester With O,o-diethylphosphorothioate
45. Schembl24839
46. Chembl1200367
47. Dtxsid1022976
48. Ecothiopate Iodide [jan]
49. Ecothiopate Iodide (jp17/inn)
50. Echothiophate Iodide [mi]
51. Ecothiopate Iodide [mart.]
52. 2-[(diethoxyphosphinyl)thio]-n,n,n-trimethylethanaminium Iodide
53. Ecothiopate Iodide [who-dd]
54. S-(2-dimethylaminoethyl)-o,o-diethylphosphorothioate Methiodide
55. (2-mercaptoethyl)trimethylammonium Iodidie O,o-diethyl Phosphorothioate
56. 2-[(diethoxyphosphoryl)sulfanyl]-n,n,n-trimethylethanaminium Iodide
57. 2-{[bis(ethyloxy)phosphoryl]thio}-n,n,n-trimethylethanaminium Iodide
58. Bdbm50016940
59. Akos015967621
60. Echothiophate Iodide [orange Book]
61. Hy-16183
62. Echothiophate Iodide [usp Impurity]
63. Echothiophate Iodide [usp Monograph]
64. D02193
| Molecular Weight | 383.23 g/mol |
|---|---|
| Molecular Formula | C9H23INO3PS |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 383.01810 g/mol |
| Monoisotopic Mass | 383.01810 g/mol |
| Topological Polar Surface Area | 60.8 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 208 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Phospholine iodide |
| PubMed Health | Echothiophate Iodide (Into the eye) |
| Drug Classes | Antiglaucoma, Miotic, Ophthalmologic Agent |
| Active Ingredient | Echothiophate iodide |
| Dosage Form | For solution |
| Route | Ophthalmic |
| Strength | 0.125% |
| Market Status | Prescription |
| Company | Wyeth Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Phospholine iodide |
| PubMed Health | Echothiophate Iodide (Into the eye) |
| Drug Classes | Antiglaucoma, Miotic, Ophthalmologic Agent |
| Active Ingredient | Echothiophate iodide |
| Dosage Form | For solution |
| Route | Ophthalmic |
| Strength | 0.125% |
| Market Status | Prescription |
| Company | Wyeth Pharms |
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Miotics
Agents causing contraction of the pupil of the eye. Some sources use the term miotics only for the parasympathomimetics but any drug used to induce miosis is included here. (See all compounds classified as Miotics.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
