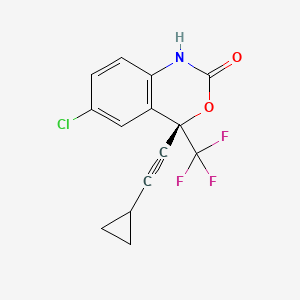

1. (s)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2h-3,1-benzoxazin-2-one
2. Dmp 266
3. Dmp-266
4. Efavirenz, (r)-isomer
5. Efavirenz, (s)-isomer
6. L 743,726
7. L 743726
8. L-743,726
9. L-743726
10. Stocrin
11. Sustiva
1. 154598-52-4
2. Sustiva
3. Stocrin
4. Dmp-266
5. Dmp 266
6. Efv
7. (4s)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2h-3,1-benzoxazin-2-one
8. (4s)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1h-3,1-benzoxazin-2-one
9. Viraday
10. Efavirenz Teva
11. L-743726
12. (rac)-efavirenz
13. L-743,726
14. (s)-efavirenz
15. (4s)-6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2h-3,1-benzoxazin-2-one
16. (s)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2h-3,1-benzoxazin-2-one
17. Nsc-742403
18. (-)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2h-3,1-benzoxazin-2-one
19. (4s)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1h-3,1-benzoxazin-2-one
20. Je6h2o27p8
21. Chebi:119486
22. Nsc742403
23. (4s)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2h-3,1-benzoxazin-2-one
24. (s)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
25. Dsstox_cid_26029
26. Dsstox_rid_81298
27. Dsstox_gsid_46029
28. (s)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2h-benzo[d][1,3]oxazin-2-one
29. (s)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1h-benzo[d][1,3]oxazin-2(4h)-one
30. 2h-3,1-benzoxazin-2-one, 6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4s)-
31. 6-chloro-4-(2-cyclopropyl-1-ethynyl)-4-trifluoromethyl-(4s)-1,4-dihydro-2h-benzo[d][1,3]oxazin-2-one
32. (-)-efavirenz
33. (rac)-dmp 266; (rac)-efv; (rac)-l-743726
34. Eravirenz
35. Smr000466351
36. Strocin (tm)
37. Sustiva (tm)
38. Sustiva (tn)
39. Cas-154598-52-4
40. L 743726
41. Hsdb 7163
42. Sr-01000759360
43. Efavirenz [usp:inn:ban]
44. Unii-je6h2o27p8
45. Efavirenzum
46. Met-sdf-1.beta. & Efavirenz
47. 1ikv
48. 1ikw
49. Efavirenz- Bio-x
50. Ncgc00159337-02
51. Efavirenz, (s)
52. Efavirenz & Plga
53. Mfcd05662344
54. Efavirenz & Ifnl1
55. Efavirenz & Ifnl2
56. Efavirenz & Ifnl3
57. Efavirenz & Il-29
58. Efavirenz [inn]
59. Efavirenz [jan]
60. Dmp-266; Efavirenz
61. Efavirenz & Il-28a
62. Efavirenz & Il-28b
63. Efavirenz [mi]
64. Efavirenz [hsdb]
65. Efavirenz [usan]
66. Efv & Interleukin 29
67. Efavirenz [vandf]
68. Efv & Interleukin 28a
69. Efv & Interleukin 28b
70. Efv & Plga
71. Efavirenz [mart.]
72. Efavirenz [usp-rs]
73. Efavirenz [who-dd]
74. Efavirenz [who-ip]
75. Efv & Ifnl1
76. Efv & Ifnl2
77. Efv & Ifnl3
78. Efavirenz (jan/usp/inn)
79. Efv & Interferon Lambda-1
80. Efv & Interferon Lambda-2
81. Efv & Interferon Lambda-3
82. Efavirenz & Interleukin 29
83. Schembl37762
84. Efavirenz [ema Epar]
85. Efavirenz & Interleukin 28a
86. Efavirenz & Interleukin 28b
87. Efavirenz Ready Made Solution
88. Mls000759465
89. Mls001424087
90. Bidd:gt0383
91. Amy229
92. Bdbm2483
93. Chembl223228
94. Dmp266
95. Efv & Il-28a
96. Efv & Il-28b
97. Efavirenz [orange Book]
98. Dtxsid9046029
99. Efavirenz, >=98% (hplc)
100. Efavirenz & Interferon Lambda-1
101. Efavirenz & Interferon Lambda-2
102. Efavirenz & Interferon Lambda-3
103. Efv & Il-29
104. Gtpl11287
105. Telura Component Efavirenz
106. Efavirenz [usp Monograph]
107. Atripla Component Efavirenz
108. Bcpp000245
109. Hms2051j08
110. Hms2090n16
111. Hms3393j08
112. Hms3713m14
113. Met-stromal Cell-derived Factor-1.beta. (human) & Efavirenz
114. Efavirenzum [who-ip Latin]
115. 2h-3,1-benzoxazin-2-one, 6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4s)-
116. Bcp27719
117. Zinc2020233
118. Tox21_111582
119. Dl-535
120. S4685
121. Efavirenz Component Of Atripla
122. Akos015894951
123. Efavirenz & Poly-lactide-co-glycolide
124. Tox21_111582_1
125. Ab21723
126. Bcp9000636
127. Ccg-101011
128. Db00625
129. Ks-5380
130. Nc00261
131. Nsc 742403
132. (s)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2h-3,1-benzoxazin-2-one
133. Efavirenz 100 Microg/ml In Acetonitrile
134. Ncgc00159337-04
135. Ncgc00159337-12
136. Ncgc00271713-05
137. Ncgc00271713-08
138. Ac-25006
139. Bc164402
140. Hy-10572
141. E0997
142. C08088
143. D00896
144. F17329
145. Ab00639956-06
146. Ab00639956-08
147. 598e524
148. A809555
149. Q422645
150. J-520431
151. Sr-01000759360-4
152. Sr-01000759360-5
153. Z2186909878
154. Efavirenz, United States Pharmacopeia (usp) Reference Standard
155. Efavirenz Solution, 1.0 Mg/ml In Acetonitrile, Certified Reference Material
156. (4s)-6-chloranyl-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1h-3,1-benzoxazin-2-one
157. 2h-3, 6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4s)-
158. (s)-6-chloro-4-(cyclopropyl-ethynyl)-1,4-dihydro-4-(trifluoromethyl)-2h-3,1-benzoxazine-2-one
159. (s)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-(s)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2h-3,1-benzoxazin-2-one4-(trifluoromethyl)-2h-3,1-benzoxazin-2-one
160. (s)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2h-3,1-ben Zoxazin-2-one
161. (s)-6-chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2h-3,1-benzoxazin-2-one
| Molecular Weight | 315.67 g/mol |
|---|---|
| Molecular Formula | C14H9ClF3NO2 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 315.0273907 g/mol |
| Monoisotopic Mass | 315.0273907 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Efavirenz |
| PubMed Health | Efavirenz (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SUSTIVA (efavirenz) is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formu... |
| Active Ingredient | Efavirenz |
| Dosage Form | Tablet; Capsule |
| Route | oral |
| Strength | 200mg; 100mg; 50mg; 600mg |
| Market Status | Tentative Approval |
| Company | Matrix Labs; Macleods Pharms; Hetero Drugs; Strides; Aurobindo; Par Formulations Private; Emcure Pharma; Aurobindo Pharma; Cipla; Micro Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Sustiva |
| PubMed Health | Efavirenz (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SUSTIVA (efavirenz) is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formu... |
| Active Ingredient | Efavirenz |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 600mg; 50mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 3 of 4 | |
|---|---|
| Drug Name | Efavirenz |
| PubMed Health | Efavirenz (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SUSTIVA (efavirenz) is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formu... |
| Active Ingredient | Efavirenz |
| Dosage Form | Tablet; Capsule |
| Route | oral |
| Strength | 200mg; 100mg; 50mg; 600mg |
| Market Status | Tentative Approval |
| Company | Matrix Labs; Macleods Pharms; Hetero Drugs; Strides; Aurobindo; Par Formulations Private; Emcure Pharma; Aurobindo Pharma; Cipla; Micro Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Sustiva |
| PubMed Health | Efavirenz (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | SUSTIVA (efavirenz) is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formu... |
| Active Ingredient | Efavirenz |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 600mg; 50mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
Anti-HIV Agents; Reverse Transcriptase Inhibitors
National Library of Medicine's Medical Subject Headings. Efavirenz. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Due to ongoing neuropsychiatric adverse events in some efavirenz (EFV)-treated patients, a switch to an alternative non-nucleoside reverse transcriptase inhibitor may be considered. Rilpivirine (RPV) has been coformulated as a single-tablet regimen (STR) with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), and the components have demonstrated noninferior efficacy to EFV+FTC/TDF, good tolerability profile, and high adherence. After discontinuation, EFV has an extended inductive effect on cytochrome P450 (CYP) 3A4 that, after switching, may reduce RPV exposures and adversely impact clinical outcomes. This study examines the clinical implications of reduced RPV exposures with concomitant FTC/TDF and declining EFV exposures when patients, intolerant to EFV, switch from EFV/FTC/TDF to RPV/FTC/TDF. This 48-week, phase 2b, open-label, multicenter study evaluated the efficacy and safety of switching from EFV/FTC/TDF (>/= 3 months duration) to RPV/FTC/TDF. Virologic suppression (HIV-1 RNA <50 copies/mL), safety, and EFV and RPV pharmacokinetics were assessed. At weeks 12 and 24, all 49 dosed subjects remained suppressed on RPV/FTC/TDF. At week 48, 46 (93.9%) subjects remained suppressed and virologic failure occurred in 2/49 (4.1%) subjects with no emergence of resistance. EFV concentrations were above the 90th percentile for inhibitory concentration (IC90) for several weeks after EFV discontinuation, and RPV exposures were in the range observed in phase 3 studies by approximately 2 weeks post switch. No subjects discontinued the study due to an adverse event. Switching from EFV/FTC/TDF to RPV/FTC/ TDF was a safe, efficacious option for virologically suppressed HIV-infected patients with EFV intolerance wishing to remain on an STR.
PMID:24144898 Mills AM et al; HIV Clin Trials 14 (5): 216-23 (2013)
Efavirenz is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1184
To report a case of acquired long QT syndrome that, after exclusion of all other possible causes, was probably related to therapy with efavirenz, a novel nonnucleoside reverse transcriptase inhibitor.
PMID:12022902 Castillo R et al; Ann Pharmacother 36 (6): 1006-8 (2002)
About 53% of adults receiving efavirenz (600 mg once daily) in controlled clinical studies reported adverse CNS effects such as abnormal dreams, abnormal thinking, agitation, amnesia, confusion, depersonalization, dizziness, euphoria, hallucinations, impaired concentration, insomnia, somnolence, and stupor; these adverse effects were reported in 25% of adults in the control groups not receiving efavirenz. These effects were described as mild (do not interfere with daily activities) in 33.3%, moderate (may interfere with daily activities) in 17.4%, or severe (interrupt usual daily activities) in 2% of patients receiving efavirenz and required discontinuance of the drug in 2.1%. Dizziness was reported in 28.1% and insomnia was reported in 16.3% of patients receiving the drug. Impaired concentration, somnolence, or abnormal dreams were reported in 6.2-8.3% and hallucinations were reported in 1.2% of patients.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 660
Serious adverse psychiatric symptoms have been reported rarely in adults receiving efavirenz. Severe depression, suicidal ideation, nonfatal suicide attempts, aggressive behavior, paranoid reactions, or manic reactions have been reported in 0.4-1.6% of patients receiving efavirenz in controlled clinical studies; these psychiatric symptoms were reported in up to 0.6% of those in the control groups not receiving the drug. The incidence of each of these psychiatric symptoms ranges from 0.3% (for manic reactions) to 2% (for severe depression or suicidal ideation) in patients with a prior history of psychiatric disorders, and these individuals appear to be at greater risk of such symptoms than other individuals. Other psychiatric symptoms reported in controlled clinical studies in adults receiving efavirenz include depression (15.8%), anxiety (11.1%), and nervousness (6.3%); these symptoms were reported in 13.1, 7.6, or 2%, respectively, of those in the control groups not receiving the drug. Although a causal relationship with efavirenz has not been established, there have been occasional postmarketing reports of death by suicide, delusions, or psychosis-like behavior in patients receiving efavirenz. In addition, aggressive reactions, agitation, emotional lability, mania, neurosis,and paranoia have been reported during postmarketing surveillance. There is no evidence that patients who develop adverse CNS effects (e.g., dizziness, insomnia, impaired concentration, abnormal dreams) during efavirenz therapy are at greater risk of developing psychiatric symptoms.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 661
Fatigue has been reported in up to 7% of adults receiving efavirenz in clinical studies. Other adverse nervous system effects reported during postmarketing surveillance include abnormal coordination, ataxia, seizures, hypoesthesia, paresthesia, neuropathy, and tremor. Adverse CNS effects occurred in 18% of children receiving efavirenz in clinical studies.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 661
For more Drug Warnings (Complete) data for EFAVIRENZ (21 total), please visit the HSDB record page.
For use in combination treatment of HIV infection (AIDS)
FDA Label
Stocrin is indicated in antiviral combination treatment of human-immunodeficiency-virus-1 (HIV-1)-infected adults, adolescents and children three years of age and older.
Stocrin has not been adequately studied in patients with advanced HIV disease, namely in patients with CD4 counts < 50 cells/mm3, or after failure of protease-inhibitor (PI)-containing regimens. Although cross-resistance of efavirenz with PIs has not been documented, there are at present insufficient data on the efficacy of subsequent use of PI-based combination therapy after failure of regimens containing Stocrin.
Efavirenz is indicated in antiviral combination treatment of human-immunodeficiency-virus-1 (HIV-1)-infected adults, adolescents and children 3 years of age and older.
Efavirenz has not been adequately studied in patients with advanced HIV disease, namely in patients with CD4 counts < 50 cells/mm3, or after failure of protease inhibitor (PI)-containing regimens. Although cross-resistance of efavirenz with protease inhibitors (PIs) has not been documented, there are at present insufficient data on the efficacy of subsequent use of PI-based combination therapy after failure of regimens containing efavirenz.
Sustiva is indicated in antiviral combination treatment of human-immunodeficiency-virus-1 (HIV-1)-infected adults, adolescents and children three years of age and older.
Sustiva has not been adequately studied in patients with advanced HIV disease, namely in patients with CD4 counts < 50 cells/mm3, or after failure of protease-inhibitor (PI)-containing regimens. Although cross-resistance of efavirenz with PIs has not been documented, there are at present insufficient data on the efficacy of subsequent use of PI-based combination therapy after failure of regimens containing Sustiva.
Efavirenz (dideoxyinosine, ddI) is an oral non-nucleoside reverse transcriptase inhibitor (NNRTI). It is a synthetic purine derivative and, similar to zidovudine, zalcitabine, and stavudine. Efavirenz was originally approved specifically for the treatment of HIV infections in patients who failed therapy with zidovudine. Currently, the CDC recommends that Efavirenz be given as part of a three-drug regimen that includes another nucleoside reverse transcriptase inhibitor (e.g., lamivudine, stavudine, zidovudine) and a protease inhibitor or efavirenz when treating HIV infection.
Cytochrome P-450 CYP2C19 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C19. (See all compounds classified as Cytochrome P-450 CYP2C19 Inhibitors.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
Cytochrome P-450 CYP2B6 Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP2B6. (See all compounds classified as Cytochrome P-450 CYP2B6 Inducers.)
Cytochrome P-450 CYP2C9 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C9. (See all compounds classified as Cytochrome P-450 CYP2C9 Inhibitors.)
J05AG03
J05AG03
J05AG03
J05AG03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AG - Non-nucleoside reverse transcriptase inhibitors
J05AG03 - Efavirenz
Route of Elimination
Nearly all of the urinary excretion of the radiolabeled drug was in the form of metabolites.
Oral bioavailability of efavirenz may be affected by administration with food. Administration of a single 600-mg dose of efavirenz as capsules with a high-fat, high-calorie meal (894 kcal, 54 g fat, 54% of calories from fat) or a reduced-fat, normal-calorie meal (440 kcal, 2 g fat, 4% of calories from fat) increases peak plasma concentrations of the drug by 39 or 51%, respectively, and AUC by 22 or 17%, respectively, compared with administration in the fasting state. Administration of a single 600-mg dose of efavirenz as tablets with a high-fat, high-calorie meal (approximately 1000 kcal, 500-600 kcal from fat) increases peak plasma concentrations and AUC of the drug by 79 and 28%, respectively, compared with administration in the fasting state.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 667
Efavirenz is excreted principally in the feces, both as unchanged drug and metabolites. Excretion of efavirenz has been evaluated in individuals receiving 400 mg daily for 1 month. Following oral administration of 400 mg of radiolabeled efavirenz on day 8, 14-34% of the dose was excreted in urine (less than 1% as unchanged drug), and 16-61% was excreted in feces (predominantly as unchanged drug).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 667
Efavirenz is about 99.5-99.75% bound to plasma proteins, principally albumin.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 667
In HIV-infected adults receiving efavirenz 200, 400, or 600 mg once daily, peak plasma concentrations of the drug generally occur in 3-5 hours and steady-state plasma concentrations are achieved in 6-10 days. Following continued administration of efavirenz, plasma concentrations are lower than expected from single-dose studies, presumably because of increased clearance of the drug. In one study in individuals receiving efavirenz 200-400 mg once daily for 10 days, plasma concentrations of the drug were 22-42% lower than those predicted from single-dose studies. Following oral administration of efavirenz 600 mg once daily in HIV-infected adults, peak plasma concentration, trough plasma concentration, and AUC of the drug at steady-state averaged 4.1 mcg/mL, 1.8 mcg/mL, and 58. mcg*hour/mL, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 666
For more Absorption, Distribution and Excretion (Complete) data for EFAVIRENZ (8 total), please visit the HSDB record page.
Efavirenz is principally metabolized by the cytochrome P450 system to hydroxylated metabolites with subsequent glucuronidation of these hydroxylated metabolites. These metabolites are essentially inactive against HIV-1.
Efavirenz was metabolized extensively by all the species as evidenced by the excretion of none or trace quantities of parent compound in urine. Significant species differences in the metabolism of efavirenz were observed. The major metabolite excreted in the urine of all species was the O-glucuronide conjugate (M1) of the 8-hydroxylated metabolite. Efavirenz was also metabolized by direct conjugation with glucuronic acid, forming the N-glucuronide (M2) in all five species. The sulfate conjugate of 8-OH efavirenz (M3) was found in the urine of rats and cynomolgus monkeys but not in humans. In addition to the aromatic ring-hydroxylated products, metabolites with a hydroxylated cyclopropane ring (at C14) were also isolated. GSH-related products of efavirenz were identified in rats and guinea pigs. The cysteinylglycine adduct (M10), formed from the GSH adduct (M9), was found in significant quantities in only rat and guinea pig urine and was not detected in other species. In vitro metabolism studies were conducted to show that the GSH adduct was produced from the cyclopropanol intermediate (M11) in the presence of only rat liver and kidney subcellular fractions and was not formed by similar preparations from humans or cynomolgus monkeys. These studies indicated the existence of a specific glutathione-S-transferase in rats capable of metabolizing the cyclopropanol metabolite (M11) to the GSH adduct, M9.
PMID:10534318 Mutlib AE et al; Drug Metab Dispos 27 (11): 1319-33 (1999)
Efavirenz is a substrate for cytochrome p450 isoforms, particularly CYP3A4 and CYP2B6. The 8-hydroxy metabolite is excreted in the urine, and the glucuronide conjugate of 8-hydroxy-efavirenz is present in plasma and urine. Sixty percent of the dose is excreted in urine as the glucuronide conjugate.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1363
Efavirenz has known human metabolites that include 8-hydroxyefavirenz.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
40-55 hours
The terminal elimination half-life of efavirenz is prolonged in patients with chronic liver disease. Following oral administration of a single 400-mg dose of efavirenz, an elimination half-life of 152 or 118 hours was reported in individuals with or without chronic liver disease, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 667
The terminal elimination half-life of efavirenz reported in single-dose studies is longer than that reported in multiple-dose studies and has averaged 52-76 hours after a single oral dose and 40-55 hours following administration of 200-400 mg daily for 10 days.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 667
Similar to zidovudine, efavirenz inhibits the activity of viral RNA-directed DNA polymerase (i.e., reverse transcriptase). Antiviral activity of efavirenz is dependent on intracellular conversion to the active triphosphorylated form. The rate of efavirenz phosphorylation varies, depending on cell type. It is believed that inhibition of reverse transcriptase interferes with the generation of DNA copies of viral RNA, which, in turn, are necessary for synthesis of new virions. Intracellular enzymes subsequently eliminate the HIV particle that previously had been uncoated, and left unprotected, during entry into the host cell. Thus, reverse transcriptase inhibitors are virustatic and do not eliminate HIV from the body. Even though human DNA polymerase is less susceptible to the pharmacologic effects of triphosphorylated efavirenz, this action may nevertheless account for some of the drug's toxicity.
Efavirenz diffuses into the cell where it binds adjacent to the active site of reverse transcriptase. This produces a conformational change in the enzyme that inhibits function.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1363