

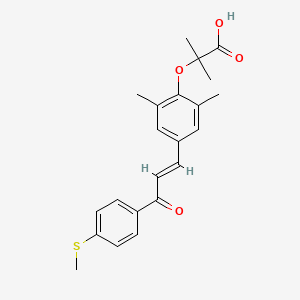

1. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxo-1-propenyl)phenoxyl)-2-methylpropanoic Acid
2. Gft505
1. 923978-27-2
2. Gft505
3. Gft-505
4. 824932-88-9
5. Elafibranor [inn]
6. Elafibranor [usan]
7. Gft 505
8. Elafibranor(gft505)
9. 2-[2,6-dimethyl-4-[(e)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]phenoxy]-2-methylpropanoic Acid
10. 2j3h5c81a5
11. Elafibranor (usan)
12. (e)-2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
13. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
14. 2-{2,6-dimethyl-4-[(1e)-3-[4-(methylsulfanyl)phenyl]-3-oxoprop-1-en-1-yl]phenoxy}-2-methylpropanoic Acid
15. Propanoic Acid, 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxo-1-propen-1-yl)phenoxy)-2-methyl-
16. Unii-2j3h5c81a5
17. Gft-505;elafibranor
18. Surecn815512
19. Elafibranor [who-dd]
20. Schembl815512
21. Chembl3707395
22. Schembl16552997
23. Gtpl11135
24. Ex-a757
25. Dtxsid601045330
26. Bcp19067
27. Zhb93288
28. Bdbm50502541
29. Mfcd27987940
30. S3720
31. Zinc114643710
32. Ccg-268462
33. Cs-5522
34. Db05187
35. (e)-2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-enyl)phenoxy)-2-methylpropanoic Acid
36. Ac-31455
37. Ac-35189
38. As-57112
39. Be163306
40. Hy-16737
41. D11208
42. A856857
43. J-690356
44. Q15409440
45. 2-(2,6-dimethyl-4-(3-(4-(methylsulfanyl)phenyl)-3-oxoprop- 1-en-1-yl)phenoxy)-2-methylpropanoic Acid
46. 2-(2,6-dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoicacid
47. 2-[2,6-dimethyl-4-[(1e)-3-[4-(methylthio)phenyl]-3-oxo-1-propen-1-yl]phenoxy]-2-methylpropanoic Acid
48. Propanoic Acid, 2-(2,6-dimethyl-4-((1e)-3-(4-(methylthio)phenyl)-3-oxo-1-propen-1-yl)phenoxy)-
49. Propanoic Acid, 2-[2,6-dimethyl-4-[(1e)-3-[4-(methylthio)phenyl]-3-oxo-1-propen-1-yl]phenoxy]-2-methyl-
| Molecular Weight | 384.5 g/mol |
|---|---|
| Molecular Formula | C22H24O4S |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 384.13953042 g/mol |
| Monoisotopic Mass | 384.13953042 g/mol |
| Topological Polar Surface Area | 88.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 537 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in atherosclerosis and diabetes mellitus type 2.
Treatment of primary biliary cholangitis
Treatment of non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH)
GFT505 is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. It has a sophisticated mechanism of action. It is able to differentially recruit cofactors to the nuclear receptor, which subsequently lead to differential regulation of genes and biological effect. Therefore, the ability to identify and profile the activity of selective nuclear receptor modulator (SNuRMs) is a powerful approach to select innovative drug candidates with improved efficacy and diminished side effects. These pluripotent and multimodal molecules have significant positive effects on obesity, insulin-resistance and diabetes, atherosclerosis, inflammation, and the lipid triad (increasing of HDL cholesterol, lowering of triglycerides and LDL cholesterol).