

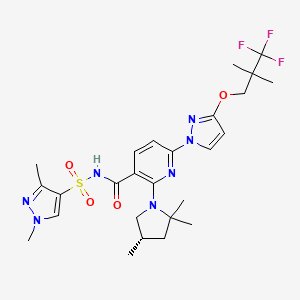

1. 3-pyridinecarboxamide, N-((1,3-dimethyl-1h-pyrazol-4-yl)sulfonyl)-6-(3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1h-pyrazol-1-yl)-2-((4s)-2,2,4-trimethyl-1-pyrrolidinyl)-
2. Vx-445
3. Vx445
1. 2216712-66-0
2. Vx-445
3. Elexacaftor [usan]
4. Rrn67gmb0v
5. Elexacaftor (usan)
6. (s)-n-((1,3-dimethyl-1h-pyrazol-4-yl)sulfonyl)-6-(3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1h-pyrazol-1-yl)-2-(2,2,4-trimethylpyrrolidin-1-yl)nicotinamide
7. 3-pyridinecarboxamide, N-((1,3-dimethyl-1h-pyrazol-4-yl)sulfonyl)-6-(3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1h-pyrazol-1-yl)-2-((4s)-2,2,4-trimethyl-1-pyrrolidinyl)-
8. N-(1,3-dimethylpyrazol-4-yl)sulfonyl-6-[3-(3,3,3-trifluoro-2,2-dimethylpropoxy)pyrazol-1-yl]-2-[(4s)-2,2,4-trimethylpyrrolidin-1-yl]pyridine-3-carboxamide
9. (6p)-n-(1,3-dimethyl-1h-pyrazole-4-sulfonyl)-6-[3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1h-pyrazol-1-yl]-2-[(4s)-2,2,4-trimethylpyrrolidin-1-yl]pyridine-3-carboxamide
10. Wjx
11. Elexacaftor [mi]
12. Unii-rrn67gmb0v
13. Elexacaftor [inn]
14. Elexacaftor (vx-445)
15. Elexacaftor [who-dd]
16. Elexacaftor/ivacaftor/tezacaftor
17. Chembl4298128
18. Schembl20239811
19. Gtpl10552
20. Elexacaftor [orange Book]
21. Dtxsid901027907
22. Ex-a3637
23. S8851
24. Trikafta Component Elexacaftor
25. Vx-445vx-445
26. Who 11180
27. At16051
28. Db15444
29. Elexacaftor Component Of Trikafta
30. Compound 1 [wo2018107100a1]
31. Ac-36746
32. Hy-111772
33. Cs-0090942
34. D11507
35. A930250
| Molecular Weight | 597.7 g/mol |
|---|---|
| Molecular Formula | C26H34F3N7O4S |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 597.23450825 g/mol |
| Monoisotopic Mass | 597.23450825 g/mol |
| Topological Polar Surface Area | 133 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Elexacaftor, in combination with [ivacaftor] and [tezacaftor] as the combination product TrikaftaTM, is indicated for the treatment of cystic fibrosis (CF) in patients 12 years of age and older who have at least one _F508del_ mutation in the CTFR gene.
As a CFTR corrector, elexacaftor works to increase the amount of mature CFTR proteins present on the surface of cells. When used in combination with CFTR potentiators, which enhance the function of cell-surface CFTR proteins, drugs like elexacaftor help to improve a variety of multi-organ cystic fibrosis symptoms, including lung function, nutritional status, and overall quality of life. TrikaftaTM, the triple combination product containing elexacaftor, may cause elevations in liver transaminases. Liver function testing should be conducted prior to beginning Trikafta, every 3 months for the first year of treatment, and annually thereafter.
Chloride Channel Agonists
A class of drugs that stimulate chloride ion influx through cell membrane channels. (See all compounds classified as Chloride Channel Agonists.)
Absorption
The absolute oral bioavailability of elexacaftor is approximately 80%. The steady-state AUC0-24h and Cmax following once daily dosing with elexacaftor 200mg are 162 mcgh/mL and 8.7 mcg/mL, respectively, and the median Tmax is 6 hours. The AUC of elexacaftor is increased 1.9-2.5-fold following a moderate-fat meal - for this reason, it is recommended to give TrikaftaTM with fat-containing food.
Route of Elimination
Approximately 87.3% of an administered radio-labeled dose of elexacaftor was found in the feces, mostly as metabolites, while only 0.23% of that same dose was found excreted in the urine.
Volume of Distribution
The apparent volume of distribution of elexacaftor is 53.7 L.
Clearance
The mean apparent clearance of elexacaftor is 1.18 L/h.
The metabolism of elexacaftor is extensive and primarily catalyzed via CYP3A4/5. Its main active metabolite, M23-ELX, carries a similar potency as the parent drug. The precise metabolic pathway of elexacaftor has not yet been elucidated in published research.
The mean terminal half-life of elexacaftor is approximately 24.7 hours.
Cystic fibrosis (CF) is the result of a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR proteins produced by this gene are transmembrane ion channels that move sodium and chloride across cell membranes - water follows the flow of chloride ions to the cell surface, which consequently helps to hydrate the surface of the cell and thin the secretions (i.e. mucous) around the cell. Mutations in the CFTR gene produce CFTR proteins of insufficient quantity and/or function, leading to defective ion transport and a build-up of thick mucous throughout the body that causes multi-organ disease involving the pulmonary, gastrointestinal, and pancreatic systems (amongst others). The most common CFTR mutation, the _F508del_ mutation, is estimated to account for 70 to 90% of all CFTR mutations and results in severe processing and trafficking defects of the CFTR protein. Elexacaftor is a CFTR corrector that modulates CFTR proteins to facilitate trafficking to the cell surface for incorporation into the cell membrane. The end result is an increase in the number of mature CFTR proteins present at the cell surface and, therefore, improved ion transport and CF symptomatology. Elexacaftor is used in combination with tezacaftor, another CFTR corrector with a different mechanism of action, and ivacaftor, a CFTR potentiator that improves the function of CFTR proteins on the cell surface - this multi-faceted, triple-drug approach confers a synergistic effect beyond that seen in typical corrector/potentiator dual therapy regimens.