


1. Cerdelga
2. Eliglustat
3. Genz-112638
1. Eliglustat Hemitartrate
2. Cerdelga
3. 928659-70-5
4. Agalsidase Beta
5. Eliglustat (hemitartrate)
6. Genz-112638
7. 104138-64-9
8. Eliglustat Tartrate [usan]
9. Eliglustat (tartrate)
10. Genz 112638
11. 928659-70-5 (tartrate)
12. N0493335p3
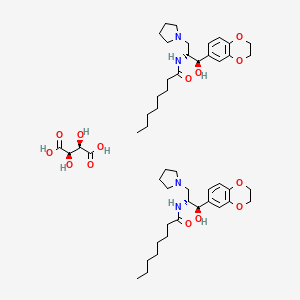
13. N-[(1r,2r)-1-(2,3-dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-pyrrolidin-1-ylpropan-2-yl]octanamide;(2r,3r)-2,3-dihydroxybutanedioic Acid
14. N-((1r,2r)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1-hydroxy-3-(pyrrolidin-1-yl)propan-2-yl)octanamide Hemi((2r,3r)-2,3-dihydroxysuccinate)
15. Unii-n0493335p3
16. Cerdelga (tn)
17. Eliglustat L-tartrate
18. Eliglustat Tartrate [mi]
19. Chebi:83353
20. Eliglustat Tartrate (jan/usan)
21. Dtxsid50239166
22. Eliglustat Tartrate [jan]
23. Eliglustat Tartrategenz-112638
24. Eliglustat Tartrate [who-dd]
25. Hy-14885a
26. Genz-112638;eliglustat Hemitartrate
27. Cs-5423
28. Ex-a2301-1
29. Eliglustat Hemitartrate (genz-112638)
30. Eliglustat Tartrate [orange Book]
31. Ac-35333
32. Bis(n-((1r,2r)-2-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(pyrrolidin-1- Ylmethyl)ethyl)octanamide) (2r,3r)-2,3-dihydroxybutanedioate
33. Octanamide, N-((1r,2r)-2-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1- Pyrrolidinylmethyl)ethyl)-, (2r,3r)-2,3-dihydroxybutanedioate (2:1)
34. Cerdelga Component Eliglustat Tartrate
35. S4433
36. C16736
37. D09894
38. Eliglustat Tartrate Component Of Cerdelga
39. Q27156779
40. (1r,2r)-octanoic Acid(2-(2',3'-dihydro-benzo(1,4) Dioxin-6'-yl)-2-hydroxy-1-pyrrolidin-1-ylmethyl-ethyl)-amide-l-tartaric Acid Salt
41. Bis{1-[(2r,3r)-3-(2,3-dihydro-1,4-benzodioxin-6-yl)-3-hydroxy-2-(octanoylamino)propyl]pyrrolidinium} (2r,3r)-2,3-dihydroxysuccinate
42. Bis{n-[(1r,2r)-1-(2,3-dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-(pyrrolidin-1-yl)propan-2-yl]octanamide} (2r,3r)-2,3-dihydroxysuccinic Acid
43. N-((1r,2r)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1-hydroxy-3-(pyrrolidin-1-yl)propan-2-yl)octanamide ((2r,3r)-2,3-dihydroxysuccinate)(2:1)
44. Octanamide, N-((1r,2r)-2-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1-pyrrolidinylmethyl)ethyl)-, (2r,3r)-2,3-dihydroxybutanedioate (2:1)
| Molecular Weight | 959.2 g/mol |
|---|---|
| Molecular Formula | C50H78N4O14 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 25 |
| Exact Mass | 958.55145317 g/mol |
| Monoisotopic Mass | 958.55145317 g/mol |
| Topological Polar Surface Area | 257 Ų |
| Heavy Atom Count | 68 |
| Formal Charge | 0 |
| Complexity | 617 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Agalsidase beta is indicated in the treatment of Fabry disease.
FDA Label
Cerdelga is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1), who are CYP2D6 poor metabolisers (PMs), intermediate metabolisers (IMs) or extensive metabolisers (EMs).
Treatment of Gaucher disease Type 1 and Type 3, Treatment of Gaucher disease Type 2
Fabrazyme is indicated for long-term enzyme replacement therapy in patients with a confirmed diagnosis of Fabry disease (-galactosidase-A deficiency).
Agalsidase beta is a recombinant human -galactosidase A used as enzyme replacement therapy in the treatment of Fabry disease. It has a long duration of action and a wide therapeutic index. Patients should be counselled regarding the risk of infusion related reactions and hypersensitivity.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
A16AX10
A16AB04
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AB - Enzymes
A16AB04 - Agalsidase beta
Absorption
A 1 mg/kg dose of agalsidase beta with a mean infusion length of 115 minutes reaches a Cmax 5.0 1.1 g/mL with an AUC of 496 137 g\*min/mL.
Route of Elimination
After nonspecific proteolysis, the amino acids from protein drugs are reused for protein synthesis or further broken down and eliminated by the kidneys.
Volume of Distribution
A 1 mg/kg dose of agalsidase beta with a mean infusion length of 115 minutes has a VSS of 112 13 mL/kg.
Clearance
A 1 mg/kg dose of agalsidase beta with a mean infusion length of 115 minutes has a clearance of 2.1 0.7 mL/min/kg.
Data regarding the metabolism of agalsidase beta is not readily available. However, protein drugs are expected to be degraded by proteases and other catalytic enzymes to smaller peptides and amino acids.
agalsidase beta has a half like of 67 12 min for a 1 mg/kg dose with a mean infusion length of 115 minutes.
-galactosidase A is uptaken by cells via the mannose 6 phosphate receptor. Agalsidase beta hydrolyzes globotriaosylceramide and other glycosphingolipids that would normally be hydrolyzed by endogenous -galactosidase A. Preventing the accumulation of glycosphingolipids prevents or reduces the severity of manifestations of Fabry disease such as renal failure, cardiomyopathy, or cerebrovascular events.