
1. Acid, Benzoaric
2. Acid, Ellagic
3. Benzoaric Acid
1. 476-66-4
2. Benzoaric Acid
3. Lagistase
4. Elagostasine
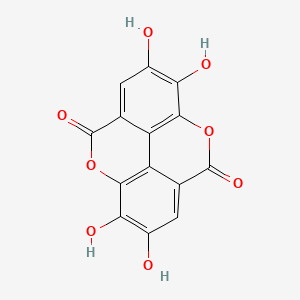
5. 2,3,7,8-tetrahydroxychromeno[5,4,3-cde]chromene-5,10-dione
6. Eleagic Acid
7. Alizarine Yellow
8. Gallogen
9. Llagic Acid
10. Acide Ellagique
11. Acido Elagico
12. Acidum Ellagicum
13. C.i. 55005
14. C.i. 75270
15. Ellagicacid
16. Ellagate
17. Gallogen, Astringent
18. Mls000069632
19. Nsc407286
20. Nsc-407286
21. Nsc-656272
22. 19yrn3zs9p
23. Chembl6246
24. Smr000058244
25. Chebi:4775
26. 4,4',5,5',6,6'-hexahydroxydiphenic Acid 2,6,2',6'-dilactone
27. 2,3,7,8-tetrahydroxy(1)benzopyrano(5,4,3-cde)(1)benzopyran-5,10-dione
28. Nsc656272
29. Tetrahydroxy[?]dione
30. Ncgc00017245-02
31. Dsstox_cid_557
32. (1)benzopyrano(5,4,3-cde)(1)benzopyran-5,10-dione, 2,3,7,8-tetrahydroxy-
33. 2,3,7,8-tetrahydroxy(1)benzopyrano(5,4,3-cde)-(1)benzopyran-5,10-dione
34. 2,3,7,8-tetrahydroxy[1]benzopyrano[5,4,3-cde][1]benzopyran-5,10-dione
35. Dsstox_rid_75657
36. Dsstox_gsid_20557
37. 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.04,16.011,15]hexadeca-1(15),4,6,8(16),11,13-hexaene-3,10-dione
38. Gallogen (van)
39. Gallogen (astringent)
40. Pomegranate Juice
41. [1]benzopyrano[5,4,3-cde][1]benzopyran-5,10-dione, 2,3,7,8-tetrahydroxy-
42. Ellagic Acid [inn:dcf]
43. Cas-476-66-4
44. Acido Elagico [inn-spanish]
45. Ccris 774
46. Acide Ellagique [inn-french]
47. Acidum Ellagicum [inn-latin]
48. Sr-01000721925
49. Einecs 207-508-3
50. Mfcd00006914
51. Unii-19yrn3zs9p
52. Nsc 407286
53. Nsc 656272
54. Brn 0047549
55. Benzoarate
56. Ellagsaeure
57. Polyphenolic
58. Eleagate
59. Ellagic
60. Llagate
61. Ellagic-acid
62. Elagic Acid
63. Hsdb 7574
64. 2zjw
65. Ref
66. Ellagic Acid, 96%
67. Spectrum_001194
68. Diphenic Acid, 4,4',5,5',6,6'-hexahydroxy-, Di-delta-lactone
69. Spectrum2_000905
70. Spectrum3_001535
71. Spectrum4_000750
72. Spectrum5_000959
73. Ellagic Acid [mi]
74. Ellagic Acid [inn]
75. Ellagic Acid [hsdb]
76. Ellagic Acid [inci]
77. Oprea1_032884
78. Schembl20429
79. Bspbio_002950
80. Kbiogr_001080
81. Kbioss_001674
82. 5-19-07-00108 (beilstein Handbook Reference)
83. Mls006011868
84. Bidd:er0482
85. Bidd:gt0565
86. Spectrum1502245
87. Spbio_000750
88. Ellagic Acid [who-dd]
89. Bdbm4078
90. Ltk-20
91. Dtxsid2020557
92. Schembl19184504
93. Bcbcmap01_000154
94. Cid_5281855
95. Kbio2_001674
96. Kbio2_004242
97. Kbio2_006810
98. Kbio3_002450
99. Ellagic Acid, Analytical Standard
100. Hms1921n20
101. Hms3673m09
102. Pharmakon1600-01502245
103. Bcp10830
104. Hy-b0183
105. Tnp00132
106. Zinc3872446
107. Tox21_110805
108. Bbl009292
109. Ccg-36358
110. Nsc758198
111. S1327
112. Stk801964
113. Ellagic Acid, >=96.0% (hpce)
114. Akos004120045
115. Tox21_110805_1
116. Cs-2067
117. Db08846
118. Nsc-758198
119. Sdccgmls-0066664.p001
120. Smp1_000111
121. Ncgc00017245-01
122. Ncgc00017245-03
123. Ncgc00017245-04
124. Ncgc00017245-05
125. Ncgc00017245-06
126. Ncgc00017245-07
127. Ncgc00017245-08
128. Ncgc00017245-09
129. Ncgc00017245-10
130. Ncgc00017245-12
131. Ncgc00094975-01
132. Ncgc00094975-02
133. Ncgc00094975-03
134. Ncgc00094975-04
135. Ncgc00178375-01
136. Ac-11647
137. Ac-30710
138. As-35095
139. Nci60_003869
140. Sbi-0051742.p002
141. E0375
142. Ellagic Acid, Dihydrate - Cas 476-66-4
143. Ft-0603405
144. Mls-0066664.0001
145. Ab00052292_11
146. Ab00052292_12
147. A872094
148. Q422044
149. Sr-01000721925-3
150. Sr-01000721925-4
151. Sr-01000721925-5
152. Sr-01000721925-6
153. Sr-01000721925-7
154. W-202834
155. Brd-k30466858-001-05-4
156. Ellagic Acid, Primary Pharmaceutical Reference Standard
157. Ellagic Acid, >=95% (hplc), Powder, From Tree Bark
158. Diphenic Acid,4',5,5',6,6'-hexahydroxy-, Di-.delta.-lactone
159. 2,3,7,8-tetrahydroxychromeno[5,4,3-cde]chromene-5,10-dione #
160. Diphenic Acid, 4,4',5,5',6,6'-hexahydroxy-, Di-.delta.-lactone
161. [1]benzopyrano[5,3-cde][1]benzopyran-5,10-dione, 2,3,7,8-tetrahydroxy-
162. 2,3,7,8-tetrahydroxy-[1]benzopyrano[5,4,3-cde][1]benzopyran-5,10-dione
163. 2,3,7,8-tetrahydroxy-chromeno[5,4,3-cde]chromene-5,10-dione
164. 2,3,7,8-tetrahydroxy[1]benzopyrano-[5,4,3-cde][1]benzopyran-5,10-dione
165. [1,2'-dicarboxylic Acid, 4,4',5,5',6,6'-hexahydroxy-, Di-.delta.-lactone
166. (1,1'-biphenyl)-2,2'-dicarboxylic Acid, 4,4',5,5',6,6'-hexahydroxy-, Di-.delta.-lactone
167. 122328-15-8
168. 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0^{4,16}.0^{11,15}]hexadeca-1(14),4(16),5,7,11(15),12-hexaene-3,10-dione
169. 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0^{4,16}.0^{11,15}]hexadeca-1(15),4,6,8(16),11,13-hexaene-3,10-dione
| Molecular Weight | 302.19 g/mol |
|---|---|
| Molecular Formula | C14H6O8 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 302.00626715 g/mol |
| Monoisotopic Mass | 302.00626715 g/mol |
| Topological Polar Surface Area | 134 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 475 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Italian researchers found that ellagic acid seemed to reduce the side effects of chemotherapy in men with advanced prostate cancer, although it did not help slow disease progression or improve survival.
American Cancer Society; Making Treatment Decisions - Ellagic Acid. (July 12, 2007) Available from, as of March 27, 2008: https://www.cancer.org/docroot/ETO/content/ETO_5_3X_Ellagic_Acid.asp?sitearea=ETO&viewmode=print&
/EXPL THER/ Ellagic acid seems to have some anti-cancer properties. It can act as an antioxidant, and has been found to cause apoptosis (cell death) in cancer cells in the lab. In other lab studies, ellagic acid seems to reduce the effect of estrogen in promoting growth of breast cancer cells in tissue cultures. There are also reports that it may help the liver to break down or remove some cancer-causing substances from the blood. Some supporters have claimed these results mean that ellagic acid can prevent or treat cancer in humans. This has not been proven. Unfortunately, many substances showing promise against cancer in lab and animal studies have not been found to be useful in people. Ellagic acid has also been said to reduce heart disease, birth defects, liver problems, and to promote wound healing. The available scientific research does not support these claims at this time.
American Cancer Society; Making Treatment Decisions - Ellagic Acid. (July 12, 2007) Available from, as of March 27, 2008: https://www.cancer.org/docroot/ETO/content/ETO_5_3X_Ellagic_Acid.asp?sitearea=ETO&viewmode=print&
/EXPL THER/ ... Ellagic acid (EA) at a dose of 4 g/kg diet inhibited multiplicity of tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mice by 54%. This inhibition was dose related between 0.06 and 4.0 g/kg diet ...
PMID:1437655 Boukharta M et al; Nutr Cancer 18(2):181-9 (1992)
/EXPL THER/ When administered in a semi-purified diet at concentrations of 0.4 and 4 g/kg, ellagic acid (EA) produced a significant (21 to 55%) decrease in the average number of N-nitrosobenzylmethylamine (NBMA)-induced esophageal tumors after 20 and 27 weeks of the bioassay. EA exhibited inhibitory effects toward preneoplastic lesions as well as neoplastic lesions. Tumors were not observed in vehicle-control rats or in rats that received EA alone.
PMID:2295128 Mandal S et al; Carcinogenesis 11(1):55-61 (1990)
/EXPL THER/ Ellagic acid inhibited lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mice. This inhibition was related to the logarithm of the dose of ellagic acid added to the diet. The biodistribution of ellagic acid was studied in mice gavaged with ellagic acid. Pulmonary levels of ellagic acid were directly proportional to the dose of ellagic acid between 0.2 and 2.0 mmol/kg ...
PMID:8512246 Castonguay A; Ann N Y Acad Sci 686:177-85 (1993)
Ellagic acid is available in supplement form, but it has not been tested for safety. Some reports indicate it may affect certain enzymes in the liver, which could alter levels of some drugs in the body. For this reason, people taking medicines or other dietary supplements should talk with their doctors or pharmacists about all their medicines and supplements before taking ellagic acid. The raspberry leaf, or preparations made from it, should be used with caution during pregnancy because they may initiate labor.
American Cancer Society; Making Treatment Decisions - Ellagic Acid. (July 12, 2007) Available from, as of March 27, 2008: https://www.cancer.org/docroot/ETO/content/ETO_5_3X_Ellagic_Acid.asp?sitearea=ETO&viewmode=print&
Ellagic acid is being investigated for use in follicular lymphoma, brain injury in intrauterine growth restricted babies, obese adolescents, and solar lentigines.
Ellagic acid's therapeutic action mostly involves antioxidant and anti-proliferative/anti-cancer effects.
Absorption
After oral consumption, ellagic acid reaches maximum concentrations in about 1 hour.
Clearance
Ellagic acid is eliminated from the body in about 4 hours.
The present study was initiated to determine ... the distribution of (14)C-ellagic acid (EA) and (3)H-N-methyl-N-nitrosourea (MNU) in the rat whole embryo culture model system ... (14)C-EA (50 uM for 2 hr, known embryoprotective concentration; no MNU added) was used to demonstrate access of EA to the embryo within the 2 hr exposure period. The majority of EA (99.5%) remained in the media while tissue concentrations of 57.0 and 47.9 pmol/mg were attained in the yolk sacs and embryos, respectively.
PMID:8322221 Frank AA et al; Teratology 47(4):275-80 (1993)
Ellagic acid (EA), derived from fruit ellagitannins, is known to be antimutagenic and anticarcinogenic in various animal tumor models. In this study, EA at a dose of 4 g/kg diet inhibited multiplicity of tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mice by 54%. This inhibition was dose related between 0.06 and 4.0 g/kg diet. In contrast, two related compounds, esculin and esculetin, had no effect on lung tumorigenesis. The biodistribution of ellagic acid (EA) /in A/J mice/ was studied as a function of dose and time after gavage of EA. The levels of EA in the lung were directly proportional to the dose of EA between 0.2 and 2.0 mmol. The maximum level of EA, corresponding to 21.3 nmol/g, was observed 30 minutes after gavage with 2.0 mmol of EA/kg body wt, which corresponds to only 70 ppm of the administered dose. The levels in liver tissues were 10-fold lower and reached a maximum 30 minutes after gavage. At this interval, the blood level of EA was 1 nmol/mL. The inclusion of EA in cyclodextrin doubles the level of EA in lung tissues. These results demonstrate that EA localizes preferentially in lung tissues ...
PMID:1437655 Boukharta M et al; Nutr Cancer 18(2):181-9 (1992)
... Ellagic acid (EA), a dietary antioxidant associated with poor biopharmaceutical properties, was encapsulated into poly(lactide-co-glycolide) (PLGA) and polycaprolactone (PCL) nanoparticles to improve oral bioavailability ... The antioxidant potential of the didodecyldimethyl ammonium bromide (DMAB)-stabilized nanoparticulate formulations was evaluated against cyclosporine A (CyA)-induced nephrotoxicity in rats ... From in situ permeation studies in rats, it was evident that intestinal uptake of EA as DMAB-stabilized nanoparticles was significantly higher as compared to the sodium carboxymethyl cellulose suspension and the polyvinyl alcohol (PVA)-stabilized particles. EA and EA nanoparticles were able to prevent the CyA-induced nephrotoxicity in rats as evident by biochemical parameters as well as kidney histopathology.
PMID:17377747 Sonaje K et al; Pharm Res 24(5):899-908 (2007)
The exact mechanism of action of ellagic acid in its different potential indications is still being investigated.
Induction of glutathione S-transferase (GST) enzymes can increase detoxification of carcinogens and reduce carcinogen-induced mutagenesis and tumorigenesis. To determine if the anticarcinogen ellagic acid induces cellular enzymes which detoxify carcinogens ... the effect of ellagic acid on the expression of glutathione S-transferase-Ya /was examined/. Rats fed ellagic acid demonstrated significant increases in total hepatic GST activity, hepatic GST-Ya activity and hepatic GST-Ya mRNA. To determine if the observed increase in GST-Ya mRNA was due to ellagic acid inducing transcription of the GST-Ya gene, transfection studies were performed with plasmid constructs containing various portions of the 5' regulatory region of the rat GST-Ya gene. The transfection studies demonstrated that ellagic acid increased GST-Ya mRNA by inducing transcription of the GST-Ya gene and demonstrated that this induction is mediated through the antioxidant responsive element of the GST-Ya gene.
PMID:7697830 Barch DH et al; Carcinogenesis 16(3):665-8 (1995)
Induction of cellular detoxification enzymes can increase detoxification of carcinogens and reduce carcinogen-induced mutagenesis and tumorigenesis. To determine if the dietary anticarcinogen ellagic acid induced enzymes which detoxify xenobiotics and carcinogens ... the effect of ellagic acid on the expression of the phase II detoxification enzyme NAD(P)H:quinone reductase (QR) /was examined/. QR is induced by xenobiotics and antioxidants interacting with the xenobiotic responsive and antioxidant responsive elements of the 5' regulatory region of the QR gene. Ellagic acid is structurally related to the antioxidants which induce QR and we proposed that ellagic acid would induce QR expression through activation of the antioxidant responsive element of the QR gene. Rats fed ellagic acid demonstrated a 9-fold increase in hepatic and a 2-fold increase in pulmonary QR activity, associated with an 8-fold increase in hepatic QR mRNA. To determine if this increase in QR mRNA was due to activation of the antioxidant responsive element, transient transfection studies were performed with plasmid constructs containing various portions of the 5' regulatory region of the rat QR gene. These transfection studies confirmed that ellagic acid induces transcription of the QR gene and demonstrated that this induction is mediated through the antioxidant responsive element of the QR gene.
PMID:7522986 Barch DH, Rundhaugen LM; Carcinogenesis 15(9):2065-8 (1994)
A principal mechanism by which these chemopreventive compounds exert their protective effects is likely to be via induction of carcinogen detoxification. This can be mediated by conjugation with glutathione, which is synthesized by the sequential actions of glutamate-cysteine ligase (GLCL) and glutathione synthetase ... /It was/ demonstrated that dietary administration of the naturally occurring chemopreventive agents, ellagic acid, coumarin or alpha-angelicalactone caused an increase in GLCL activity of between approximately 3- and 5-fold in rat liver. Treatment with the synthetic antioxidant ethoxyquin or the classic inducer phenobarbital caused < 2-fold induction of GLCL activity in rat liver, which was not found to be significant. The increases in GLCL activity were accompanied by increases (between 2- and 4-fold) in levels of both the catalytic heavy subunit (GLCLC) and regulatory light subunit (GLCLR). No substantial induction of GLCL was observed in rat kidney. The glutathione S-transferase (GST) subunits A1, A3, A4, A5, P1 and M1 were all found to be inducible in rat liver by most of the agents. The greatest levels of induction were observed for GST P1, following treatment with coumarin (20-fold), alpha-angelicalactone (10-fold) or ellagic acid (6-fold), and GST A5, following treatment with coumarin (7-fold), alpha-angelicalactone (6-fold) and ethoxyquin (6-fold). Glutathione synthetase was induced approximately 1.5-fold by coumarin, alpha-angelicalactone, ellagic acid and ethoxyquin. The expression of glutathione-related enzymes was also examined in preneoplastic lesions induced in rat liver by aflatoxin B(1). The majority of gamma-glutamyltranspeptidase (GGT)-positive preneoplastic foci contained increased levels of GLCLC relative to the surrounding tissue. This was usually found to be accompanied by an increase in GLCLR. Cells in the inner cortex of rat kidney were found to contain the highest levels of both GLCLC and GLCLR. The same cells showed the strongest staining for GGT activity.
PMID:11023540 Shepherd AG et al; Carcinogenesis 21(10):1827-34 (2000)
... Specific in vitro assays ... found ellagic acid ... to be /a/ potent inhibitors of the catalytic activities of the two /human DNA/ topoisomerases. The minimum concentration required to inhibit > or = 50% of catalytic activity (IC50) of ellagic acid was determined at 0.6 and 0.7 ug/mL for topo I and topo II, respectively ... Unlike topoisomerase poisons ... /the/ plant phenol did not trap the enzyme-DNA reaction intermediate, known as the cleavable complex. In contrast, ellagic acid prevented other topo I and topo II poisons from stabilizing the cleavable complex, suggesting that the mode of its action is that of an antagonist. Structure-activity studies identified the 3,3'-hydroxyl groups and the lactone groups as the most essential elements for the topoisomerase inhibitory actions of plant phenols. On the basis of these findings and other properties of ellagic acid, a mechanistic model for the documented anticarcinogenic effects of the agent is proposed.
PMID:7644381 Constantinou A et al; Nutr Cancer 23(2):121-30 (1995)