


1. Adolquir
2. Badyket
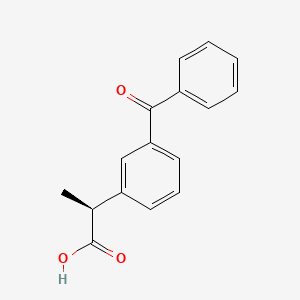
3. Dexketoprofen Trometamol
4. Enangel
5. Enantyum
6. Keral
7. Ketesse
8. Quiralam
9. Quirgel
10. Sympal
1. (s)-(+)-ketoprofen
2. 22161-81-5
3. (s)-ketoprofen
4. (+)-ketoprofen
5. (s)-2-(3-benzoylphenyl)propanoic Acid
6. (2s)-2-(3-benzoylphenyl)propanoic Acid
7. S-(+)-ketoprofen
8. Dexketoprofen [inn]
9. Arveles
10. (+)-3-benzoylhydratropic Acid
11. (+)-(s)-m-benzoylhydratropic Acid
12. Hydratropic Acid, M-benzoyl-, (+)-
13. Ketoprofen, (s)-
14. (s)-3-benzoyl-alpha-methylbenzeneacetic Acid
15. Chembl75435
16. (s)-(+)-2-(3-benzoylphenyl)propionic Acid
17. Chebi:76128
18. 6kd9e78x68
19. Dexketoprofen (inn)
20. (2s)-2-[3-(benzenecarbonyl)phenyl]propanoic Acid
21. Smr000857177
22. Dexketoprofen [inn:ban]
23. Unii-6kd9e78x68
24. 9kl
25. Mfcd00673316
26. Biomolki_000007
27. Biomolki2_000017
28. Schembl66987
29. Bmk1-b7
30. Mls001333189
31. Mls001333190
32. Benzeneacetic Acid, 3-benzoyl-alpha-methyl-, (s)-
33. Dexketoprofen [who-dd]
34. Zinc5560
35. (s)-(+)-ketoprofen, 99%
36. Dtxsid40905141
37. Hms2090m22
38. Hms2234m14
39. Bcp13810
40. Hy-b2137
41. Bdbm50088570
42. S5192
43. 2-(3-benzoyl-phenyl)-propionic Acid
44. Akos015913672
45. Ac-8103
46. Ccg-100611
47. Cs-8173
48. Db09214
49. Ncgc00142585-01
50. Ncgc00142585-02
51. As-17683
52. Bk166230
53. (s)-2-(3-benzoyl-phenyl)-propionic Acid
54. (+)-2-(3-benzoylphenyl)propionic Acid
55. (s)-(+)-2-(3-benzoylphenyl) Propionic Acid
56. (s)-(+)-3-benzoyl-?-methylbenzeneacetic Acid
57. D07269
58. D94685
59. (2s)-2-(3-benzoylphenyl)propionic Acid
60. Ab00918363-05
61. (s)-(+)-3-benzoyl-alpha-methylbenzeneacetic Acid
62. 161k815
63. A878675
64. Q425440
65. S-(+)-ketoprofen; (s)-ketoprofen; Dexketoprofen
66. W-201922
67. (.alpha.s)-3-benzoyl-.alpha.-methylbenzeneacetic Acid
68. Benzeneacetic Acid, 3-benzoyl-.alpha.-methyl-, (.alpha.s)-
69. Benzeneacetic Acid, 3-benzoyl-.alpha.-methyl-, (s)-
70. Benzeneacetic Acid, 3-benzoyl-?-methyl-, (s)-hydratropic Acid, M-benzoyl-, (+)-
| Molecular Weight | 254.28 g/mol |
|---|---|
| Molecular Formula | C16H14O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 254.094294304 g/mol |
| Monoisotopic Mass | 254.094294304 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For short-term treatment of mild to moderate pain, including dysmenorrhoea, musculoskeletal pain and toothache.
This drug is an isomer of ketoprofen. Dexketoprofen a propionic acid derivative with analgesic, anti-inflammatory, and antipyretic properties.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE17 - Dexketoprofen
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA27 - Dexketoprofen
Absorption
After oral ingestion, the Dexketoprofen onset of action is within 30 minutes. The plasma half-life of Dexketoprofen is about 4-6 hours. The Cmax is about 30 minutes
Route of Elimination
Approximately 70 to 80% of the ingested dose is recovered in the urine during the first 12 hours post-ingestion, mainly as the acyl-conjugated form of the drug.
Volume of Distribution
<0.25 L/kg
Clearance
Mainly cleared via glucuronide conjugation and followed by renal excretion, mainly unchanged.
Dexketoprofen is highly lipophilic, and is metabolized in the liver by glucuronidation. In one study, after oral administration of 25 mg of dexketoprofen to young healthy adults, Tmax was approximately 30 min for a Cmax of 3.7 0.72 mg/l. Dexketoprofen trometamol is metabolized by the hepatic cytochrome P450 enzymes (CYP2C8 and CYP2C9). Dexketoprofen trometamol has a number of metabolites, with hydroxyl derivatives making up the greatest volume. In humans, hydroxylation plays a minor role. Dexketoprofen is primarily conjugated to an acyl-glucuronide
1.65 h
It is a non-steroidal anti-inflammatory drug (NSAID) that reduces prostaglandin synthesis via inhibition of cyclooxygenase pathway (both COX-1 and COX-2) activity.