

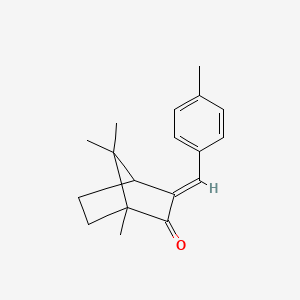

1. 3-(4'-methylbenzylidene)camphor
2. 3-(4-methylbenzylidene)camphor
3. 4-mbc Cpd
4. 4-methylbenzylidene Camphor
5. Eusolex 6300
6. Eusolex-6300
1. 3-(4'-methylbenzylidene)camphor
2. 4-methylbenzylidene Camphor
3. 38102-62-4
4. 36861-47-9
5. 3-(4-methylbenzylidene)camphor
6. Parsol 5000
7. 4-methylbenzylidenecamphor
8. 3-(4-methylbenzyliden)camphor
9. 3-(4-methylbenzylidene)-camphor
10. 3-(p-methylbenzylidene)-dl-camphor
11. 4-mbc
12. 1782069-81-1
13. Methyl Benzylidene Camphor
14. Enzacamene D-l Form
15. (e)-1,7,7-trimethyl-3-(4-methylbenzylidene)bicyclo[2.2.1]heptan-2-one
16. (e)-enzacamene
17. (+/-)-3-(p-methylbenzylidene)camphor
18. Schembl83245
19. Chembl2104261
20. Chebi:135937
21. Dtxsid201348972
22. Bdbm50103609
23. Akos015865884
24. Akos025310897
25. Ac-8477
26. Db11219
27. Ncgc00167419-02
28. Ls-14659
29. Q4637174
30. 1,7,7-trimethyl-3-[(e)-4-methylbenzylidene]bicyclo[2.2.1]heptane-2-one
31. (e)-rac-1,7,7-trimethyl-3-(4-methyl-benzylidene)-bicyclo-[2.2.1]-heptan-2-one
| Molecular Weight | 254.4 g/mol |
|---|---|
| Molecular Formula | C18H22O |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 254.167065321 g/mol |
| Monoisotopic Mass | 254.167065321 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 423 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for use as an active sunscreen agent.
Several studies suggest that enzacamene elicit estrogen-like effects. In prepubertal male rats exposed to enzacamene during embryonic and fetal development, decrease in testicular weight with decreased levels of LH, GnRH, and glutamate were observed; in comparison, there was an increase in LH, GnRH, and aspartate levels in peripubertal rats. These findings suggest that high concentrations of enzacamene during embryonic and fetal stage inhibits the testicular axis in male rats during the prepubertal stage and stimulates it during peripubertad stage. In a study of zebrafish (Danio rerio) embryo, exposure to enzacamene during early vertebrate development was associated with muscular and neuronal defects that may result in developmental defects, including a reduction in AChE activity, disorganized pattern of slow muscle fibers, and axon pathfinding errors during motor neuron innervation. Enzacamene displays a weak binding activity in receptors binding assays using the mammalian estrogen receptor (ER).
Absorption
The maximum plasma concentration of enzacamene was 16ng/mL in healthy female volunteers following daily whole-body topical application of 2mg/cm^2 of sunscreen formulation at 10% (weight/weight) for four days. Blood concentration of enzacamene (4-MBC) and its main metabolite, 3-(4-carboxybenzylidene)camphor, peaked within 10 h after oral administration of enzacamene.
Route of Elimination
The urine concentration of 4 ng/mL and 4 ng/mL of enzacamene were observed in female and male volunteers, respectively. In a rat pharmacokinetic study, most of orally administered enzacamene was recovered in in feces as 3-(4-carboxybenzylidene)camphor and, to a smaller extent, as 3-(4-carboxybenzylidene)-6-hydroxycamphor. Glucuronides of both metabolites were also detectable in faces. In urine, one isomer of 3-(4-carboxybenzylidene)hydroxycamphor was the predominant metabolite [3-(4-carboxybenzylidene)-6-hydroxycamphor], the other isomers and 3-(4-carboxybenzylidene)camphor were only minor metabolites excreted with urine. Enterohepatic circulation of glucuronides derived from the two major 4-MBC metabolites may explain the slow excretion of 4-MBC metabolites with urine and the small percentage of the administered doses recovered in urine.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
Based on the findings of a rat pharmacokinetic study, it is proposed that absorbed enzacamene following oral administration undergo extensive first-pass hepatic metabolism. Following oral administration of enzacamene (4-MBC) in rats, detected metabolites in the plasma and urine were 3-(4-carboxybenzylidene)camphor and as four isomers of 3-(4-carboxybenzylidene)hydroxycamphor containing the hydroxyl group located in the camphor ring system with 3-(4-carboxybenzylidene)-6-hydroxycamphor as the major metabolite. However the blood concentrations of 3-(4-carboxybenzylidene)-6-hydroxycamphor were below the limit of detection following peak concentration. Via hydroxylation mediated by cytochrome P450 system, 3-(4-hydroxymethylbenzylidene)camphor is formed. This metabolite is further oxidized to 3-(4-carboxybenzylidene)camphor via oxidation of alcohol dehydrogenase and aldehyde dehydrogenase, and may be further hydroxylated to form 3-(4-carboxybenzylidene)-6-hydroxycamphor mediated by CYP450 system.
The half life of enzacamene (4-MBC) and its main metabolite, 3-(4-carboxybenzylidene)camphor, displayed half-lives of approximately 15 h after reaching peak plasma concentrations after oral administration in rats.
Enzacamene absorbs UV-B rays. It is proposed that enzacamene exerts estrogen-like activities in the same direction as endogenous estrogens via nonclassical estrogen signaling mechanisms that do not involve gene regulation by the nuclear ER. It binds to cytosolic estradiol binding sites of estrogen receptors with low to moderate affinity compared to that of the endogenous agonist. Based on the findings of a study with _Xenopus_ hepatocytes in culture, enzacamene has a potential to induce the ER gene only at higher concentrations (10100 mol/L). While enzacamene was not shown to activate estrogen-dependent gene transcription when tested in an ER reporter gene assay in yeast cells, it was demonstrated in _Xenopus_ hepatocytes cultures that activate ER-dependent signaling mechanisms leading to altered gene expression. In micromolar concentrations, enzacamene accelerates cell proliferation rate in MCF-7 human breast cancer cells.