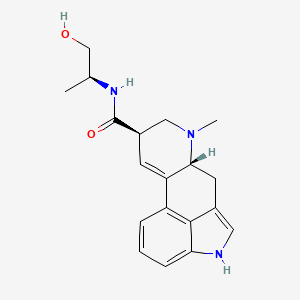

1. Ergobasin
2. Ergometrin
3. Ergometrine Maleate
4. Ergonovine
5. Ergonovine Maleate
6. Ergotrate
1. Ergonovine
2. Ergobasine
3. Ergotocine
4. Ergostetrine
5. 60-79-7
6. Margonovine
7. Ergometrinum
8. Ergometrina
9. Ergotrate
10. Ergometrin
11. D-lysergic Acid-l-propanolamide
12. D-lysergic Acid 1-hydroxymethylethylamide
13. Ergoklinine
14. Secacornin
15. Secometrin
16. Ergotrate Maleate
17. N-(alpha-(hydroxymethyl)ethyl)-d-lysergamide
18. Ergometrine (inn)
19. 9,10-didehydro-n-(2-hydroxy-1-methylethyl)-6-methylergoline-8beta(s)-carboxamide
20. 9,10-didehydro-n-(alpha-(hydroxymethyl)ethyl)-6-methylergoline-8-beta-carboxamide
21. Lysergic Acid Propanolamide
22. Chebi:4822
23. Wh41d8433d
24. (6ar,9r)-n-[(2s)-1-hydroxypropan-2-yl]-7-methyl-6,6a,8,9-tetrahydro-4h-indolo[4,3-fg]quinoline-9-carboxamide
25. Ergometrine [inn]
26. Ergometrine [inn:ban]
27. [8beta(s)]-9,10-didehydro-n-(2-hydroxy-1-methylethyl)-6-methylergoline-8-carboxamide
28. N-(2-hydroxy-1-methylethyl)-d-(+)-lysergamide
29. (6ar,9r)-n-((s)-1-hydroxypropan-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide
30. Ergometrina [inn-spanish]
31. Lysergamide, N-((s)-2-hydroxy-1-methylethyl)-
32. Ergometrinum [inn-latin]
33. (6ar,9r)-7-methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]quinoline-9-carboxylic Acid ((s)-2-hydroxy-1-methyl-ethyl)-amide
34. L-lysergic-l(beta-hydroxyisopropylamide)
35. Hsdb 4075
36. N-(1-(hydroxymethyl)ethyl)-d-lysergamide
37. Einecs 200-485-0
38. N-(2-hydroxy-1-methylethyl)-d(+)-lysergamide
39. Unii-wh41d8433d
40. Ncgc00163165-01
41. Ergonovine [mi]
42. Ergonovine [hsdb]
43. Ergonovine [vandf]
44. Dsstox_cid_26323
45. Dsstox_rid_81539
46. Ergoline-8-carboxamide, 9,10-didehydro-n-(2-hydroxy-1-methylethyl)-6-methyl-, (8beta(s))-
47. Dsstox_gsid_46323
48. Schembl78181
49. Ergometrine [who-dd]
50. Gtpl148
51. Chembl119443
52. Dtxsid8046323
53. 9,10-didehydro-n-((s)-2-hydroxy-1-methylethyl)-6-methylergoline-8.beta.-carboxamide
54. Ergoline-8-carboxamide, 9,10-didehydro-n-(2-hydroxy-1-methylethyl)-6-methyl-, (8.beta.(s))-
55. Tox21_112022
56. Bdbm50390991
57. Zinc53174604
58. Akos024282585
59. Db01253
60. Cas-60-79-7
61. Ergoline-8-beta-carboxamide, 9,10-didehydro-n-((s)-2-hydroxy-1-methylethyl)-6-methyl-
62. N-[(2s)-1-hydroxypropan-2-yl]-6-methyl-9,10-didehydroergoline-8beta-carboxamide
63. Smp1_000118
64. Ncgc00017253-03
65. Ft-0625687
66. C07543
67. D07905
68. Q424508
69. (4r,7r)-n-[(2s)-1-hydroxypropan-2-yl]-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide
70. (9r)-n-((s)-1-hydroxypropan-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide
| Molecular Weight | 325.4 g/mol |
|---|---|
| Molecular Formula | C19H23N3O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 325.17902698 g/mol |
| Monoisotopic Mass | 325.17902698 g/mol |
| Topological Polar Surface Area | 68.4 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 535 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oxytocics
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Ergonovine maleate and methylergonovine maleate are used for the prevention and treatment of postpartum hemorrhage caused by uterine atony. The drugs appear to be equally effective for these purposes; however, many clinicians prefer methylergonovine to ergonovine because the former drug may produce hypertension less frequently than does the latter drug. /Not included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Ergonovine maleate has been used as a provocative test for the diagnosis of variant angina. The drug has been used to precipitate coronary artery spasm in patients with suspected variant angina. /Not included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Ergonovine and methylergonovine should not be used for the induction or augmentation of labor.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
MEDICATION (VET): Oxytocic ... /used/ in control of postpartum uterine hemorrhage, removal of fluid from atonic uteri, to help prevent prolapsed uteri, and judiciously in terms of timing to aid in suturing uterus after cesarean section or in replacing everted uterus.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 197
When administered in correct doses to carefully selected patients who are closely monitored, there is little risk of serious adverse systemic effects in patients receiving ergonovine or methylergonovine.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Hypertension may occur following administration of ergonovine or methylergonovine, especially when administered iv undiluted or too rapidly or when used in conjunction with regional anesthesia or vasoconstrictors; hypertension may occur less frequently with methylergonovine than with ergonovine. Some patients, especially eclamptic or previously hypertensive patients, may be unusually sensitive to the hypertensive effects of the drugs; generalized headaches, severe arrhythmias, seizures, and cerebrovascular accidents have been associated with ergonovine- or methylergonovine-induced hypertension in these patients. Hypotension also has been reported.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Since ergonovine or methylergonovine may cause serious adverse cardiovascular effects, some clinicians recommend that the drugs not be used in patients with hypertension, heart disease, venoatrial shunts, mitral valve stenosis, or obliterative vascular disease.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Because prolonged use of ergonovine or methylergonovine may produce ergotism in sensitive individuals, prolonged use of the drugs should be avoided.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
For more Drug Warnings (Complete) data for ERGONOVINE (14 total), please visit the HSDB record page.
4 OR 5. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG; BETWEEN 1 TEASPOON & 1 OZ FOR 70 KG PERSON (150 LB). 5= EXTREMELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-50 MG/KG; BETWEEN 7 DROPS & 1 TEASPOON FOR 70 KG PERSON (150 LB). /ERGOT/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-146
Used to treat postpartum haemorrhage and postabortion haemorrhage in patients with uterine atony.
Ergonovine belongs to the group of medicines known as ergot alkaloids. These medicines are usually given to stop excessive bleeding that sometimes occurs after abortion or a baby is delivered. They work by causing the muscle of the uterus to contract.
Oxytocics
Drugs that stimulate contraction of the myometrium. They are used to induce LABOR, OBSTETRIC at term, to prevent or control postpartum or postabortion hemorrhage, and to assess fetal status in high risk pregnancies. They may also be used alone or with other drugs to induce abortions (ABORTIFACIENTS). Oxytocics used clinically include the neurohypophyseal hormone OXYTOCIN and certain prostaglandins and ergot alkaloids. (From AMA Drug Evaluations, 1994, p1157) (See all compounds classified as Oxytocics.)
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02A - Uterotonics
G02AB - Ergot alkaloids
G02AB03 - Ergometrine
Absorption
Absorption is rapid and complete after oral or intramuscular administration.
Route of Elimination
Thought to be eliminated by non-renal mechanisms (i.e. hepatic metabolism, excretion in feces)
Ergonovine maleate and methylergonovine maleate are rapidly absorbed after oral or im administration.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Uterine contractions are usually initiated within 5-15 minutes following oral administration, within 2-5 minutes after im injection, and immediately following iv injection of ergonovine or methylergonovine. Uterine contractions persist for 3 hours or longer after oral or im administration and for 45 minutes after iv injection of either drug.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Little is known about the elimination of ergonovine or methylergonovine. It has been suggested that the drugs are principally eliminated by nonrenal mechanisms (i.e., metabolism in the liver, excretion in feces).
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
The aim of this investigation was to assess the pharmacokinetics and bioavailability of ergometrine in six human male subjects after an oral dose of 0.200 mg and after an intravenous dose of 0.075 mg of ergometrine maleate. A large variation in bioavailability of between 34% and 117% in the six volunteers was observed. The lag time was also subject dependent and ranged between 0.0073 hr (0.4 min) and 0.47 hr (28 min). After intravenous administration, the pharmacokinetic profile can be described by a two-compartment model. The distribution half-life t1/2 alpha is 0.18 +/- 0.20 hr, the elimination half-life t1/2 beta is 2.06 +/- 0.90 hr, the total body clearance (CL) amounts to 35.9 +/- 13.4 L hr-1 and the steady-state volume (Vss) of distribution is 73.4 +/- 22.01. After oral administration, the pharmacokinetic profile can be described by a one-compartment model. The absorption half-life t1/2 abs is 0.19 +/- 0.22 hr, and the elimination half-life t1/2 beta 1.90 +/- 0.16 hr. This study with oral ergometrine shows such a large interindividual variability in bioavailability that the oral route of administration does not seem not to be the most reliable means of accurate dosing in preventing post-partum hemorrhage.
PMID:8161717 de Groot AN et al; Biopharm Drug Dispos 15 (1): 65-73 (1994)
Hepatic.
t1/2 α=10 minutes; t1/2 β=2 hours
The aim of this investigation was to assess the pharmacokinetics and bioavailability of ergometrine in six human male subjects after an oral dose of 0.200 mg and after an intravenous dose of 0.075 mg of ergometrine maleat. ... After intravenous administration, the pharmacokinetic profile can be described by a two-compartment model. The distribution half-life t1/2 alpha is 0.18 +/- 0.20 hr, the elimination half-life t1/2 beta is 2.06 +/- 0.90 hr ... After oral administration, the pharmacokinetic profile can be described by a one-compartment model. The absorption half-life t1/2 abs is 0.19 +/- 0.22 hr, and the elimination half-life t1/2 beta 1.90 +/- 0.16 hr. ...
PMID:8161717 de Groot AN et al; Biopharm Drug Dispos 15 (1): 65-73 (1994)
Plasma concentrations of methylergonovine appear to decline in a biphasic manner. ... Following iv administration of ergonovine to adults, the half-life of the drug in the initial phase (t1/2 alpha) reportedly is about 10 minutes and the half-life in the terminal phase (t1/2 beta) is about 2 hours.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Ergonovine directly stimulates the uterine muscle to increase force and frequency of contractions. With usual doses, these contractions precede periods of relaxation; with larger doses, basal uterine tone is elevated and these relaxation periods will be decreased. Contraction of the uterine wall around bleeding vessels at the placental site produces hemostasis. Ergonovine also induces cervical contractions. The sensitivity of the uterus to the oxytocic effect is much greater toward the end of pregnancy. The oxytocic actions of ergonovine are greater than its vascular effects. Ergonovine, like other ergot alkaloids, produces arterial vasoconstriction by stimulation of alpha-adrenergic and serotonin receptors and inhibition of endothelial-derived relaxation factor release. It is a less potent vasoconstrictor than ergotamine. As a diagnostic aid (coronary vasospasm), ergonovine causes vasoconstriction of coronary arteries.
Ergonovine maleate and methylergonovine maleate are pharmacologically similar. Both drugs directly stimulate contractions of uterine and vascular smooth muscle. Following administration of usual therapeutic doses of ergonovine or methylergonovine, intense contractions of the uterus are produced and are usually followed by periods of relaxation. Larger doses of the drugs, however, produce sustained, forceful contractions followed by only short or no periods of relaxation. The drugs increase the amplitude and frequency of uterine contractions and uterine tone which in turn impede uterine blood flow. Ergonovine and methylergonovine also increase contractions of the cervix.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Ergonovine and methylergonovine produce vasoconstriction, mainly of capacitance vessels; increased central venous pressure, elevated blood pressure, and, rarely, peripheral ischemia and gangrene may result. Methylergonovine reportedly may interfere with prolactin secretion, but this effect has not been definitely established.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
... The effect of /Ergonovine/ on isolated canine tracheobronchial smooth muscle was analyzed to investigate the mechanism of ergonovine-induced airway smooth muscle contraction. Both ergonovine and serotonin (5-hydroxytryptamine, 5HT) contracted canine tracheal smooth muscle with EC50 1.4 X 10(-8) M and 5.1 X 10(-7) M, respectively. The maximal contractile response observed with ergonovine was approximately 30% less than that observed with 5HT. In diverse blockers such as methysergide, atropine, prazosin, propranolol, pyrilamine and cimetidine, only methysergide competitively blocked both ergonovine and 5HT responses with a similar calculated dissociation constant (pKB); 8.3 +/- 0.2 against ergonovine and 8.5 +/- 0.2 against 5HT (n = 6, p = 0.9). The relative affinity and efficacy of ergonovine and 5HT were determined by use of a partial irreversible antagonist, phenoxybenzamine. The calculated affinity of ergonovine was about 16 times higher than that of 5HT. The relative efficacy of EC100 for ergonovine was 0.2 but at EC10 it was 41.9 (5HT efficacy = 1). Ergonovine 10(-9) M or 10(-8) M shifted the 5HT dose-response curve to the right without reducing the maximal response, but the shift was nonparallel. Ergonovine and 5HT also contracted canine bronchial smooth muscle in a dose dependent manner with EC50 6.4 X 10(-8) M and 1.8 X 10(-7) M, respectively. The dose-responses curve of these two agonists were competitively blocked by methysergide 10(-6) M. These data indicate that ergonovine directly contracts canine tracheobronchial smooth muscle as a result of its combination with 5HT receptors. ...
PMID:2796062 Sakamoto Y; Nihon Kyobu Shikkan Gakkai Zasshi 27 (6): 679-88 (1989)
Although commonly classified as alpha-adrenergic blocking agents, most important effects of all ergot alkaloids are due to action on CNS and direct stimulation of smooth muscle. Latter occurs in many different organs... In some organs stimulation ... shown to be due to action on same receptors involved in responses to catecholamines. Thus ergot alkaloids can be considered to be series of partial agonists with blocking activity... /Ergot Alkaloids/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 540
For more Mechanism of Action (Complete) data for ERGONOVINE (7 total), please visit the HSDB record page.