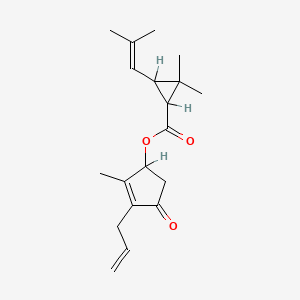

1. Allethrins
2. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester
1. 584-79-2
2. Pynamin
3. Esbiothrin
4. D-cis,trans-allethrin
5. 84030-86-4
6. Allethrine
7. D-cis-trans-allethrin
8. D-allethrin
9. Alpha-dl-trans-allethrin
10. Pallethrine
11. Allethrins
12. Pyresin
13. Pyresyn
14. Allethrin I
15. Allyl Cinerin I
16. 22431-63-6
17. Necarboxylic Acid
18. D-t-allethrin
19. D-trans-allethrin
20. Wasp Killer Ii
21. (2-methyl-4-oxo-3-prop-2-enylcyclopent-2-en-1-yl) 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate
22. Bioaletrina
23. Cinerin I Allyl Homolog
24. 3-allyl-2-methyl-4-oxo-2-cyclopenten-1-yl Chrysanthemate
25. Chebi:34572
26. Trans-chrysanthemummonocarboxylate
27. Allyl Homolog Of Cinerin I
28. D-allethrolone Chrysanthemumate
29. 42534-61-2
30. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester
31. Fmc 249
32. Ncgc00163953-03
33. Nia 249
34. Fda 1446
35. Ru 16121
36. 3972-20-1
37. (+/-)-allerethonyl (+/-)-cis,trans-chrysanthemate
38. (+)-allethronyl (+)-trans-chrysanthemumate
39. 28434-00-6
40. Ai 3-29024
41. (+)-allelrethonyl (+)-cis,trans-chrysanthemate
42. 3-allyl-4-keto-2-methylcyclopentenyl Chrysanthemum Monocarboxylate
43. Mgk Allethrin Concentrate
44. Allethrolone Ester Of Chrysanthemummonocarboxylic Acid
45. Bioallethrin (ban)
46. (+)-trans-allethrin
47. D-trans Allethrin
48. 3-allyl-2-methyl-4-oxocyclopent-2-en-1-yl 2,2-dimethyl-3-(2-methylprop-1-en-1-yl)cyclopropanecarboxylate
49. D-trans-(1-methyl-2-allyl-3-oxo-1-cyclopenten-5-yl)chrysanthemumate
50. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, (1s)-2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester, (1r,3r)-
51. Bioallathrin
52. 0x03ii877m
53. Allyl Cinerin
54. Allethrin 1
55. S-trans-bioallethrin
56. (+)-trans-bioallethrin
57. Allethrin [hsdb]
58. Allethrin I [mi]
59. Dsstox_cid_15180
60. Dsstox_rid_79245
61. Duocide [veterinary] (tn)
62. Dsstox_gsid_35180
63. Schembl26963
64. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester, (1-alpha(s*),3-beta)-(+-)-
65. Chembl1872535
66. Dtxsid8035180
67. D,l-chrysanthemum Monocarboxylate
68. [(1s)-2-methyl-4-oxo-3-prop-2-enylcyclopent-2-en-1-yl] (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate
69. Hy-b1559
70. Nsc11782
71. Tox21_400074
72. D-allethrolone D-trans-chrysanthemate
73. Ent 16275
74. Ent 17510
75. Esbiol Concentrate 90% (salt/mix)
76. Mfcd00045443
77. Allethrin 100 Microg/ml In Methanol
78. Akos015900219
79. Allethrin 1000 Microg/ml In N-hexane
80. Ncgc00163953-01
81. Ncgc00163953-02
82. Ncgc00163953-04
83. Ncgc00164471-01
84. (3-allyl-2-methyl-4-oxo-cyclopent-2-en-1-yl) 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
85. As-11751
86. Cas-584-79-2
87. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, (1r)-2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester. (1s.3s)-rel-
88. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester, [1r-[1.alpha.(s*),3.beta.]]-
89. Db-047389
90. Cs-0013441
91. Ft-0630540
92. Ft-0647652
93. Allethrin, Pestanal(r), Analytical Standard
94. Esbiothrin, Pestanal(r), Analytical Standard
95. D07530
96. Bioallethrin, Pestanal(r), Analytical Standard
97. 584a792
98. J-014709
99. Q1851694
100. W-105386
101. (.+-.)-allelrethonyl (.+-.)-cis,trans-chrysanthemate
102. (+)-trans-chrysanthemumic Acid Ester Of (.+-.)-allethrolone
103. Wln: L5v Butj B2u1 C1 Dov- Bl3tj A1 A1 C1uy1&1
104. 2-allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one Ester Of 2,2-dimethyl-3-(2-methyl Propenyl) Cyclopropane Carboxylic Acid
105. 2-methyl-4-oxo-3-(prop-2-en-1-yl)cyclopent-2-en-1-yl 2,2-dimethyl-3-(2-methylprop-1-en-1-yl)cyclopropanecarboxylate
106. 3-allyl-2-methyl-4-oxo-2-cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate, (1r-(1.alpha.(s*),3.beta.))-
107. 3-allyl-2-methyl-4-oxocyclopent-2-enyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
108. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methyl-1-propenyl)-, (1r)-2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester, (1s,3s)-rel-
109. Cyclopropanecarboxylic Acid, 2,2-dimethyl-3-(2-methylpropenyl)-, Ester With 2-allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one
110. Cyclopropanecarboxylic Acid,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester (va
111. Cyclopropanecarboxylic Acid,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl Ester, D-trans-
112. Cyclopropanecarboxylic Acid,2-dimethyl-3-(2-methyl-1-propenyl)-, 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopentene-1-yl Ester
113. Cyclopropanecarboxylic Acid,2-dimethyl-3-(2-methylpropenyl)-, Ester With 2-allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one
114. Cyclopropanecarboxylicacid, 2,2-dimethyl-3-(2-methyl-1-propen-1-yl)-,2-methyl-4-oxo-3-(2-propen-1-yl)-2-cyclopenten-1-yl Ester, (1r,3r)-
115. D-trans-allethrin, (s)-3-allyl-2-methyl-4-oxocyclopent-2-enyl-(1r,3r)-2,2-dimethyl-3-(2-methyl-prop-1-enyl)cyclopropancarboxylate
| Molecular Weight | 302.4 g/mol |
|---|---|
| Molecular Formula | C19H26O3 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 302.18819469 g/mol |
| Monoisotopic Mass | 302.18819469 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pyrethrins with piperonyl butoxide are used for topical treatment of pediculosis (lice infestations). Combinations of pyrethrins with piperonyl butoxide are not effective for treatment of scabies (mite infestations). Although there are no well-controlled comparative studies, many clinicians consider 1% lindane to be pediculicide of choice. However, some clinicians recommend use of pyrethrins with piperonyl butoxide, esp in infants, young children, & pregnant or lactating women ... . If used correctly, 1-3 treatments ... are usually 100% effective ... Oil based (eg, petroleum distillate) combinations ... produce the quickest results. ... For treatment of pediculosis, enough gel, shampoo, or solution ... should be applied to cover affected hair & adjacent areas ... After 10 min, hair is ... washed thoroughly ... treatment should be repeated after 7-10 days to kill any newly hatched lice. /Pyrethrins/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3203
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
WHEN RADIOACTIVE PYRETHROID IS ADMIN ORALLY TO MAMMALS, IT IS ABSORBED FROM INTESTINAL TRACT OF THE ANIMALS & DISTRIBUTED IN EVERY TISSUE EXAMINED. EXCRETION OF RADIOACTIVITY IN RATS ADMIN TRANS-ISOMER: DOSAGE: 500 MG/KG; INTERVAL 20 DAYS; URINE 36%; FECES 64%; TOTAL 100%. /PYRETHROIDS/
PMID:789062 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475089 MIYAMOTO J; ENVIRON HEALTH PERSPECT 14: 15-28 (1976)
Pyrethrins are absorbed through intact skin when applied topically. When animals were exposed to aerosols of pyrethrins with piperonyl butoxide being released into the air, little or none of the combination was systemically absorbed. /Pyrethrins/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3203
Although limited absorption may account for the low toxicity of some pyrethroids, rapid biodegradation by mammalian liver enzymes (ester hydrolysis and oxidation) is probably the major factor responsible. Most pyrethroid metabolites are promptly excreted, at least in part, by the kidney. /Pyrethroids/
U.S. Environmental Protection Agency/Office of Prevention, Pesticides, and Toxic Substances. Reigart, J.R., Roberts, J.R. Recognition and Management of Pesticide Poisonings. 5th ed. 1999. EPA Document No. EPA 735-R-98-003, and available in electronic format at: https://www.epa.gov/pesticides/safety/healthcare, p. 87
There were no major /metabolic/ differences between sexes, between low and high dose groups, nor between single-dose groups and repeated dose groups. The majority of radioactivity was eliminated within 3 days. Urinary elimination ranged from approximately 25 - 50% and fecal elimination ranged from 50 - 70%. There was no bioaccumulation of residue in tissues. ... /d-trans-Allethrin/
USEPA/OPPTS; Allethrins: Revised HED Chapter of Reregistration Eligibility Decision Document (RED). p.14 EPA -HQ-OPP-2006-0986-0012 (June 2007). Available from, as of June 9, 2008: https://www.regulations.gov/search/Regs/home.html#home
When allethrin labelled with (14)C in the acid moiety or with (3)H in the alcohol moiety was administered orally to male Sprague Dawley rats at levels ranging from 1 to 5 mg/kg body weight, the radiocarbon and tritium from the acid- and alcohol-labellings were eliminated in the urine (30% and 20.7%, respectively) and feces (29% and 27%, respectively) in 48 hr. ... Most of the metabolites excreted in the urine were ester-form metabolites together with two hydrolyzed products, chrysanthemum dicarboxylic acid (CDCA) and allethrolone. ...
WHO; Environ Health Criteria 87: ALLETHRINS - Allethrin - d-Allethrin - Bioallethrin - S-Bioallethrin (1989). Available from, as of June 10, 2008: https://www.inchem.org/documents/ehc/ehc/ehc87.htm
AFTER ADMINISTRATION OF LABELED ALLETHRIN TO MALE RATS, THE MAJOR METABOLITES FOUND WERE ALCOHOL-ACIDS. FROM NMR AND MASS SPECTRA A THIRD METABOLITE WAS IDENTIFIED AS ALLETHRIN WITH ONE CYCLOPROPANE METHYL HYDROXYLATED AND OXIDATION OF THE TRANSMETHYL TO A CARBOXYL GROUP. ...
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 307
/IN STUDYING THE METABOLISM OF ALLETHRIN IN HOUSEFLIES, IT WAS FOUND THAT IN/ ALLETHRIN LABELED IN THE KETOCYCLOPENTENYL PORTION OF THE MOLECULE, A METABOLITE THAT BEHAVED AS KETOCYCLOPENTENOL WAS ISOLATED BY PAPER CHROMATOGRAPHY. ... INVESTIGATORS USING ALLETHRIN LABELED IN CHRYSANTHEMUMIC ACID PORTION OF MOLECULE WERE ABLE TO DETECT ONLY TRACES OF ACID IN HOUSEFLY HOMOGENATES OR EXCRETA. ... ONLY TRACES OF UNCHANGED ALLETHRIN WERE RECOVERABLE AND THE BULK OF THE RECOVERED MATERIAL MUST BE A DERIVATIVE OF THE INTACT ESTER OR OF THE ACID.
White-Stevens, R. (ed.). Pesticides in the Environment: Volume 1, Part 1, Part 2. New York: Marcel Dekker, Inc., 1971., p. 218
Allethrin is oxidized not only at the chrysanthemate isobutenyl moiety to the corresponding primary alcohol but also at the allyl group to 1'-hydroxyprop-2'-enyl and 2',3'-dihydroxy-propyl derivatives, or at a methyl group on the cyclopropyl moiety to a hydroxy derivative. Allethrin is also converted to chrysanthemum dicarboxylic acid and allethrolone.
Aizawa, H. Metabolic Maps of Pesticides. New York, NY: Academic Press, 1982., p. 184
When allethrin was applied topically to houseflies, chromatography indicated the presence of allethrone and chrysanthemic acid in addition to allethrin and three unidentified compounds.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 470
For more Metabolism/Metabolites (Complete) data for ALLETHRINS (10 total), please visit the HSDB record page.
Mode of Action: The allethrins are a type I pyrethroid (i.e., lacking a cyano group at the alpha carbon position of the alcohol moiety). The allethrins are axonic poisons that block the closing of the sodium gates in the nerves, and, thus, prolong the return of the membrane potential to its resting state leading to hyperactivity of the nervous system which can result in paralysis and/or death.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision for Allethrins p.5 EPA 738-R-07-001 (June 2007). Available from, as of June 7, 2008: https://www.epa.gov/pesticides/reregistration/status.htm
The mechanisms by which pyrethroids alone are toxic are complex and become more complicated when they are co-formulated with either piperonyl butoxide or an organophosphorus insecticide, or both, as these compounds inhibit pyrethroid metabolism. The main effects of pyrethroids are on sodium and chloride channels. Pyrethroids modify the gating characteristics of voltage-sensitive sodium channels to delay their closure. A protracted sodium influx (referred to as a sodium 'tail current') ensues which, if it is sufficiently large and/or long, lowers the action potential threshold and causes repetitive firing; this may be the mechanism causing paraesthesiae. At high pyrethroid concentrations, the sodium tail current may be sufficiently great to prevent further action potential generation and 'conduction block' ensues. Only low pyrethroid concentrations are necessary to modify sensory neurone function.
PMID:16180929 Bradberry SM et al; Toxicol Rev 24 (2): 93-106 (2005)
The interactions of natural pyrethrins and 9 pyrethroids with the nicotinic acetylcholine (ACh) receptor/channel complex of Torpedo electronic organ membranes were studied. None reduced (3)H-ACh binding to the receptor sites, but all inhibited (3)H-labeled perhydrohistrionicotoxin binding to the channel sites in presence of carbamylcholine. Allethrin inhibited binding noncompetitively, but (3)H-labeled imipramine binding competitively, suggesting that allethrin binds to the receptor's channel sites that bind imipramine. The pyrethroids were divided into 2 types according to their action: type A, which included allethrin, was more potent in inhibiting (3)H-H12-HTX binding and acted more rapidly. Type B, which included permethrin, was less potent and their potency increased slowly with time. The high affinities that several pyrethroids have for this nicotinic ACh receptor suggest that pyrethroids may have a synaptic site of action in addition to their well known effects on the axonal channels.
Abbassy MA et al; Pestic Biochem Physiol 19 (3): 299-308 (1983)
Phosphoinositide breakdown in guinea pig cerebral cortical synaptoneurosomes induced by the Type I pyrethroids allethrin, resmethrin, and permethrin and the Type II pyrethroid deltamethrin and fenvalerate were investigated with various receptor agonists as well as sodium channel blockers and agents. Phosphoinositide breakdown was determined from inositol-phosphate formation by tritiated inositol labeled synaptoneurosomes. All five pyrethroids dose dependently induced phosphoinositide breakdown. Type II pyrethroids exhibited higher potency and deltamethrin was more efficacious than the Type I pyrethroids. Five micromolar tetrodotoxin, a blocker of voltage dependent sodium channels, partially inhibited deltamethrin (85%) and fenvalerate (60%) responses but not allethrin or resmethrin. Fenvalerate induced stimulation of phosphoinositide breakdown was additive with stimulation elicited by the receptor agonists carbamylcholine (1 mM) and norepinephrine (1000 uM) but less than additive with the sodium channel agents batrachotoxin, pumiliotoxin-B, and scorpion venom. Allethrin (100 uM) was less than additive with receptor agonists or sodium channel agents and actually significantly inhibited response to scorpion venom. Effects for 100 uM allethrin with either fenvalerate or deltamethrin were not different from allethrin alone. Ten micromolar allethrin slightly decreased response to 10 to 100 uM deltamethrin. The local anesthetic dibucaine, a sodium channel activation inhibitor, completely blocked deltamethrin induced phosphoinositide breakdown but was much less effective in inhibiting allethrin response. It appears likely that Type-I pyrethroids induce phosphoinositide breakdown through a mechanism other than sodium channel activation while Type-II pyrethroids act in a manner analogous to other sodium channel agents.
PMID:2546657 Gusovsky F et al; Brain Research 492 (1/2): 72-8 (1989)
For more Mechanism of Action (Complete) data for ALLETHRINS (13 total), please visit the HSDB record page.