
1. Ethchlorovynol
2. Ethchlorvinol
3. Placidyl
1. Alvinol
2. Ethchlorovynol
3. Placidyl
4. Arvynol
5. Ethchlorvinol
6. Ethclorvynol
7. Roeridorm
8. Normoson
9. Serensil
10. Nostel
11. Etchlorvinolo
12. Ethchlorvinyl
13. Ethochlorvynol
14. Ethychlorvynol
15. Aethyl-chlorvynol
16. Normonson
17. Normosan
18. Placidil
19. Serenesil
20. Serenil
21. Etclorvinol
22. Ethchlorvynolum
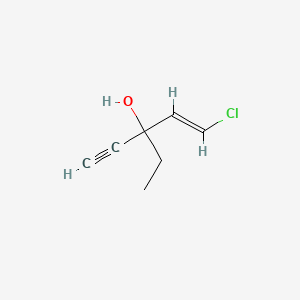
23. 1-chloro-3-ethyl-1-penten-4-yn-3-ol
24. 113-18-8
25. 1-penten-4-yn-3-ol, 1-chloro-3-ethyl-
26. 3-(beta-chlorovinyl)-1-pentyn-3-ol
27. Beta-chlorovinyl Ethyl Ethynyl Carbinol
28. Ethyl Beta-chlorovinyl Ethynyl Carbinol
29. A 71
30. Ethchlorvynol Civ
31. Nsc 30372
32. 1-chloro-3-ethylpent-1-en-4-yn-3-ol
33. (e)-1-chloro-3-ethylpent-1-en-4-yn-3-ol
34. 6eim3851uz
35. Nsc-30372
36. Serensiloline
37. Ethyl .beta.-chlorovinyl Ethynyl Carbinol
38. Nromoson
39. 1-chloro-3-ethyl-pent-1-en-4-yn-3-ol
40. (1e)-1-chloro-3-ethylpent-1-en-4-yn-3-ol
41. Etclorvinol [inn-spanish]
42. Ethchlorvynolum [inn-latin]
43. Placidyl (tn)
44. Beta-chlorvinyl Ethyl Ethynyl Carbinol
45. 1-chloro-3-ethylpent-1-4-yn-3-ol
46. 5-chloro-3-ethylpent-1-yn-4-en-3-ol
47. Hsdb 3079
48. Brn 1702245
49. Unii-6eim3851uz
50. Ai3-23721
51. Ethchlorvynol (jan/usp/inn)
52. Ethchlorvynol [usp:inn:ban]
53. Dea No. 2540
54. 3-(.beta.-chlorovinyl)-1-pentyn-3-ol
55. .beta.-chlorovinyl Ethyl Ethynyl Carbinol
56. Ethchlorvynol [mi]
57. Chembl591
58. Ethchlorvynol [inn]
59. Ethchlorvynol [jan]
60. Ethchlorvynol [hsdb]
61. Ethchlorvynol [vandf]
62. Ethchlorvynol [mart.]
63. Schembl113589
64. Schembl113591
65. Ethchlorvynol [who-dd]
66. Chebi:4882
67. Gtpl7180
68. Ethchlorvynol [orange Book]
69. Ethchlorvynol Civ [usp-rs]
70. Ethchlorvynol [usp Monograph]
71. Akos015903758
72. Db00189
73. 1-chloro-3-ethyl-1-penten-4-yn-3-ol #
74. 3-(.beta.-chlorvinyl)-1-pentyn-3-ol
75. C07833
76. D00704
77. Q3085140
78. 1-penten-4-yn-3-ol, 1-chloro-3-ethyl-, (1e)-
79. 1135334-09-6
| Molecular Weight | 144.60 g/mol |
|---|---|
| Molecular Formula | C7H9ClO |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 144.0341926 g/mol |
| Monoisotopic Mass | 144.0341926 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 154 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Used as a hypnotic in the short-term treatment of simple insomnia for periods up to 1 week in duration. /Use is included in the labeling approved by the US Food and Drug Administration/.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1364
The primary Medication Classification of the US Veterans Administration is CN309: Sedatives/Hypnotics, Other.
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IA p.1360 (1992)
ETHCHLORVYNOL IS SEDATIVE-HYPNOTIC DRUG WITH RAPID ONSET & SHORT DURATION OF ACTION ... HAS ANTICONVULSANT & MUSCLE RELAXANT PROPERTIES.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 365
BECAUSE OF A REPORTED EFFECT TO SUPRESS THE ANTICIPATED RESPONSE TO DICUMAROL, DRUG SHOULD BE USED CAUTIOUSLY IN COMBINATION WITH DRUGS METABOLIZED BY LIVER, & IT IS CONTRAINDICATED IN INTERMITTENT PORPHYRIA.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 365
...SHOULD BE WARNED AGAINST OPERATING MOTOR VEHICLE OR HAZARDOUS MACHINERY WHILE ON DRUG. ... IT SHOULD NOT BE USED IN PATIENT WITH HISTORY OF DRUG ABUSE, & DRUG SHOULD BE GRADUALLY WITHDRAWN FROM PATIENT TAKING EXCESSIVE QUANTITIES. ... SAFE & EFFECTIVE USE OF THIS AGENT DURING PREGNANCY & IN PEDIATRIC-AGE PATIENTS ... NOT ... ESTABLISHED.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1005
WITHDRAWAL SYMPTOMS MAY RESEMBLE TO DELIRIUM TREMENS, AND ARE SOMETIMES SUGGESTIVE OF SCHIZOPHRENIC REACTION. THEY ARE ESPECIALLY SEVERE IN ELDERLY PATIENTS.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 365
Ethchlorvynol crosses the placenta. ... FDA Pregnancy Category C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well controlled studies in humans, but potential benefits may warrant use ... in pregnant women despite potential risks.
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IA p.1360 (1992)
For more Drug Warnings (Complete) data for ETHCHLORVYNOL (13 total), please visit the HSDB record page.
The lethal dose usually ranges from 10 to 25 g, but death has followed a dose of 2.5 g in the presence of ethanol.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 365
Used for short-term hypnotic therapy in the management of insomnia for periods of up to one week in duration; however, this medication generally has been replaced by other sedative-hypnotic agents.
Ethchlorvynol is a sedative drug and schedule IV (USA) controlled substance. It produces cerebral depression, however the exact mechanism of action is not known.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CM - Other hypnotics and sedatives
N05CM08 - Ethchlorvynol
Absorption
Rapidly absorbed from gastrointestinal tract.
... SLOW DISAPPEARANCE OF DRUG WAS SHOWN TO BE DUE LARGELY TO EXTENSIVE TISSUE DISTRIBUTION.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 443
AFTER ORAL INGESTION OF 1 G OF ETHCHLORVYNOL, SYMPTOMS OF CNS DEPRESSION OCCUR WITHIN 15-30 MIN. VOL OF DISTRIBUTION IS GREATER THAN VOL OF TOTAL BODY WATER. IN ACUTELY INTOXICATED PATIENT ... CONCN IN CSF REACHES 50% OF THAT IN PLASMA.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 129
DRUG IS MAINLY /METABOLIZED BY THE/ LIVER, BUT APPROX 10% OF USUAL DOSE IS EXCRETED INT URINE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 129
RAPIDLY ABSORBED FROM GI TRACT ... ORAL ADMIN OF 200 MG, 500 MG & 750 MG ... PRODUCED PEAK PLASMA LEVELS OF 0.9 TO 2.5 MCG/ML, 4.2 TO 6.5 MCG/ML & 8 MCG/ML, RESPECTIVELY, WITHIN 1 TO 2 HR ... /HUMAN/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1975
For more Absorption, Distribution and Excretion (Complete) data for ETHCHLORVYNOL (17 total), please visit the HSDB record page.
About 90% of a dose is metabolized in the liver. Some ethchlorvynol may also be metabolized in the kidneys. Ethchlorvynol and metabolites undergo extensive enterohepatic recirculation.
About 90% of a dose is metabolized in the liver. Some ethchlovynol may also be metabolized in the kidneys.
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IA p.1360 (1992)
Although the metabolic fate of ethchlorvynol has not been fully elucidated, there is evidence that the drug is extensively metabolized, probably in the liver. There is also some evidence that the kidneys may play an essential role in metabolism of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1365
Metabolism in liver originally was assumed to be negligible, but this has been somewhate disproved since it was later found that ethchlorvynol is readily metabolized in liver. Although metabolites have not yet been identified, it would appear that glucuronide conjugation occurs as well as hydroxylation with reduction of unsaturated bonds in the chemical structure.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 826
Approximately 90% of the drug will undergo biotransformation, probably in the liver, since ethchlorvynol is concentrated in bile to three times as much as in serum.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 826
Plasma half-life is approximately 10 to 20 hours, terminal half-life is 21-100 hours.
AFTER EQUILIBRIUM FOLLOWING HYPNOTIC DOSES, BLOOD LEVEL FALLS RAPIDLY, WITH AVG T/2 OF 5.6 HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 129
... HALF-LIFE FOR ... DISTRIBUTION ... 5 TO 6 HR ... HALF-LIFE FOR ... ELIMINATION /IS/ 70 HR /HUMAN/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1975
Plasma half-life is approximately 10 to 20 hours.
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IA p.1360 (1992)
100 hr (overdose) /From table/
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 942
A 33 year old obese, hypothyroid, white male with several medical problems was admitted to University Hospital in September 1984 for treatment of drug intoxication. Admitting medications included ethchlorvynol in addition to other central nervous system depressants. Initial serum concn were reported at 70 ug/ml in this somnolent yet totally conscious adult. Established therapeutic concn are 2-8 ug/ml, with toxic exceeding 20 ug/ml. A tolerance phenomenon seemed evident. Serum ethchlorvynol concn were monitored daily during early hospitalization and continued to be substantially greater than reported toxic concn. Kinetic values were as follows: total body clearance 9.92 ml/min, volume of distribution 68.0 liters, and half-life 78 hr. These values are unique in that they were calculated from a patient who had not suffered an acute overdose, thereby differing markedly from previously published values. The influence of hypothyroidism and hyperlipidemia on these markedly different values appears to be significant. Ethchlorvynol should probably be added to the list of drugs influenced by thyroid disease.
PMID:3822854 Kolpek JH et al; Pharmacotherapy 6 (6): 323-7 (1986)
Although the exact mechanism of action is unknown, ethchlorvynol appears to depress the central nervous system in a manner similar to that of barbiturates. Barbiturates bind at a distinct binding sites associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.
MOST OF MODERN SEDATIVE-HYPNOTIC AGENTS ARE GENERAL DEPRESSANTS. THEY DEPRESS WIDE RANGE OF CELLULAR FUNCTIONS IN MANY VITAL ORGAN SYSTEMS. /HYPNOTICS & SEDATIVES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 102
The mechanism of action of the drug is not known. In doses used for hypnosis, ethchlorvynol produces cerebral depression and quiet, deep sleep. Higher doses may lead to general anesthesia and concurrent depression of respiratory and vasomotor centers; death may result from respiratory failure, hypotension, or complications of prolonged coma. The effect of ethchlorvynol or rapid eye movement (REM) or other stages of sleep has not yet been established. In experimental animals, ethchlorvynol exhibits anticonvulsant and muscle relaxant activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1364
Intravenous injection of ethchlorvynol (ECV) leads to hypoxemia and a permeability pulmonary edema. Whether the hypoxemia is directly attributable to the pulmonary edema or caused by release of mediators has not been explored. Three groups of dogs were studied: (1) ethchlorvynol, (2) indomethacin--ethchlorvynol, and (3) ketanserin--ethchlorvynol. In group 1, 25 to 30 mg/kg of ethchlorvynol caused a significant fall in PaO2 at 4 min (92 +/- 12.6 to 77 +/- 21 mm Hg, p <0.05), which persisted throughout the experiment. The P(A-a)O2 gradient widened significantly at 3 min (22 +/- 11 to 31 +/- 16.8 mm Hg, p <0.05) and remained abnormal for the remainder of the experiment. There was no significant fall in PaO2 in groups 2 and 3. Lung tissue water to dry weight ratio increased significantly in all groups at 60 min. Lung tissue water to dry weight ratios were normal at 10 min after ethchlorvynol injection in additional groups. It was concluded that ethchlorvynol causes hypoxemia, which is mediated by cyclooxygenase products and 5-hydroxytryptamine. This hypoxemia can be prevented by the administration of drugs that block these products.
PMID:3096135 Millen JE, Glauser FL; Am J Med Sci 292 (5): 293-8 (1986)