
1. Emeside
2. Ethosuccimid
3. Ethylmethylsuccimide
4. Ethymal
5. Etosuximida Faes
6. Faes, Etosuximida
7. Petnidan
8. Pyknolepsinum
9. Suksilep
10. Suxilep
11. Zarontin
1. 77-67-8
2. Zarontin
3. Etosuximida
4. Pyknolepsinum
5. 2-ethyl-2-methylsuccinimide
6. Ethosuxide
7. 3-ethyl-3-methylpyrrolidine-2,5-dione
8. Ethosuccimide
9. Ethosuccinimide
10. Etosuximid
11. Petnidan
12. Suxinutin
13. Atysmal
14. Emeside
15. Ethymal
16. Suxilep
17. Pentinimid
18. Peptinimid
19. Petinimid
20. Succimitin
21. Zaraondan
22. Capitus
23. Mesentol
24. Pemalin
25. Simatin
26. Succimal
27. Suximal
28. Thetamid
29. Zarodan
30. Zarondan
31. Zartalin
32. Asamid
33. Etomal
34. Pemal
35. Ronton
36. Suxin
37. Aethosuximide
38. Piknolepsin
39. Thilopemal
40. Epileo Petit Mal
41. Zarondan-saft
42. Ethosuximidum
43. Simatin(e)
44. 3-ethyl-3-methyl-2,5-pyrrolidinedione
45. 3-methyl-3-ethylsuccinimide
46. 2-methyl-2-ethylsuccinimide
47. Ci-366
48. 2,5-pyrrolidinedione, 3-ethyl-3-methyl-
49. Pm-671
50. Succinimide, 2-ethyl-2-methyl-
51. 3-ethyl-3-methylsuccinimide
52. Cn-10,395
53. Pm 671
54. 3-methyl-3-ethylpyrrolidine-2,5-dione
55. Alpha-ethyl-alpha-methylsuccinimide
56. Alpha-methyl-alpha-ethylsuccinimide
57. Gamma-methyl-gamma-ethyl-succinimide
58. C.i. 366
59. H 940
60. H-490
61. Cl 366
62. Cn-10395
63. (+-)-2-ethyl-2-methylsuccinimide
64. Nsc-64013
65. Gamma-ethyl-gamma-methyl-succinimide
66. 3-ethyl-3-methylpyrroline-2,5-dione
67. Chembl696
68. .alpha.-ethyl-.alpha.-methylsuccinimide
69. 5seh9x1d1d
70. 3-ethyl-3-methyl-pyrrolidine-2,5-dione
71. Chebi:4887
72. Aethosuccimidum
73. Etosuximide
74. Nsc64013
75. (+/-)-2-ethyl-2-methylsuccinimide
76. .gamma.-methyl-.gamma.-ethylsuccinimide
77. Etosuccimide
78. Etosuccimide [dcit]
79. Aethosuximide [german]
80. Dsstox_cid_3019
81. N-ethyl Methylsuccinimide
82. Dsstox_rid_76832
83. Dsstox_gsid_23019
84. Ethosuximidum [inn-latin]
85. Etosuximida [inn-spanish]
86. Pyknole.psi.num
87. Piknole.psi.n
88. Zarontin (tn)
89. Hsdb 1119
90. Sr-01000075863
91. Einecs 201-048-7
92. Ci 366
93. Nsc 64013
94. Unii-5seh9x1d1d
95. Brn 0117054
96. Cas-77-67-8
97. Ncgc00016320-01
98. Prestwick_611
99. Mfcd00072123
100. Ethosuximide [usan:usp:inn:ban:jan]
101. Spectrum_001385
102. Prestwick0_000165
103. Prestwick1_000165
104. Prestwick2_000165
105. Prestwick3_000165
106. Spectrum2_001483
107. Spectrum3_000944
108. Spectrum4_001051
109. Spectrum5_001073
110. Ethosuximide [mi]
111. E0746
112. (.+/-.)-ethosuximide
113. Ethosuximide [inn]
114. Ethosuximide [jan]
115. 2, 3-ethyl-3-methyl-
116. E 7138
117. Ethosuximide [hsdb]
118. Ethosuximide [usan]
119. Ethosuximide [vandf]
120. Nciopen2_000014
121. Lopac0_000532
122. Schembl34212
123. Bspbio_000029
124. Ethosuximide [mart.]
125. Kbiogr_001342
126. Kbioss_001865
127. 5-21-09-00595 (beilstein Handbook Reference)
128. Divk1c_000218
129. Ethosuximide [usp-rs]
130. Ethosuximide [who-dd]
131. Ethosuximide [who-ip]
132. Spectrum1502196
133. 2,5-pyrrolidinedione, 3-ethyl-3-methyl-, (+-)-
134. Spbio_001465
135. Spbio_001950
136. Bpbio1_000033
137. Gtpl7182
138. Wln: T5vmvtj D2 D1
139. Dtxsid7023019
140. Schembl20541518
141. Ethosuximide (jp17/usp/inn)
142. Ethosuximide, Analytical Standard
143. Hms500k20
144. Kbio1_000218
145. Kbio2_001865
146. Kbio2_004433
147. Kbio2_007001
148. Kbio3_002008
149. Ninds_000218
150. Ethosuximide [orange Book]
151. Hms1568b11
152. Hms1921l14
153. Hms2092d20
154. Hms2095b11
155. Hms3261l05
156. Hms3712b11
157. Hms3885d12
158. Pharmakon1600-01502196
159. Ethosuximide [ep Monograph]
160. Hy-b1378
161. Ethosuximide [usp Monograph]
162. Ethosuximide 1.0 Mg/ml In Methanol
163. Tox21_110370
164. Tox21_500532
165. Bdbm50240424
166. Ccg-39217
167. Ethosuximidum [who-ip Latin]
168. Nsc758192
169. S4626
170. Akos005261742
171. Tox21_110370_1
172. Cs-7976
173. Db00593
174. Lp00532
175. Nsc-758192
176. Sdccgsbi-0050515.p004
177. .alpha.-methyl-.alpha.-ethylsuccinimide
178. Idi1_000218
179. Ncgc00015418-02
180. Ncgc00015418-03
181. Ncgc00015418-04
182. Ncgc00015418-05
183. Ncgc00015418-06
184. Ncgc00015418-08
185. Ncgc00015418-09
186. Ncgc00015418-15
187. Ncgc00093923-01
188. Ncgc00093923-02
189. Ncgc00093923-03
190. Ncgc00093923-04
191. Ncgc00261217-01
192. 3-ethyl-3-methyl-2, 5-pyrrolidinedione
193. As-16859
194. Sbi-0050515.p003
195. 2, 5-pyrrolidinedione, 3-ethyl-3-methyl-
196. Ab00052288
197. Eu-0100532
198. Ft-0668060
199. Ft-0668061
200. C07505
201. D00539
202. D70258
203. Ab00052288_04
204. Zarontin3-ethyl-3-methyl-pyrrolidine-2,5-dione
205. Q421567
206. Sr-01000075863-1
207. Sr-01000075863-3
208. Sr-01000075863-5
209. W-109273
210. 3-ethyl-3-methyl-pyrrolidine-2,5-dione(ethosuximide)
211. Brd-a99633051-001-04-7
212. Brd-a99633051-001-05-4
213. 3-ethyl-3-methyl-pyrrolidine-2,5-dione (ethosuximide)
214. Z2379802739
215. 3-ethyl-5-hydroxy-3-methyl-3,4-dihydro-2h-pyrrol-2-one
216. 4-ethyl-5-hydroxy-4-methyl-3,4-dihydro-2h-pyrrol-2-one
217. 2,5-pyrrolidinedione, 3-ethyl-3-methyl-, (+/-)-
218. Ethosuximide, European Pharmacopoeia (ep) Reference Standard
219. Ethosuximide, United States Pharmacopeia (usp) Reference Standard
220. Ethosuximide Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 141.17 g/mol |
|---|---|
| Molecular Formula | C7H11NO2 |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 141.078978594 g/mol |
| Monoisotopic Mass | 141.078978594 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 188 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Ethosuximide |
| PubMed Health | Ethosuximide (By mouth) |
| Drug Classes | Anticonvulsant |
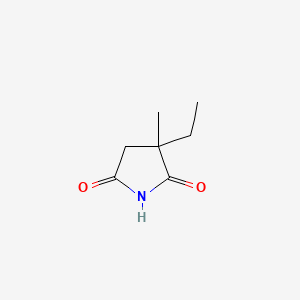
| Drug Label | Ethosuximide is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:Each ethosuximide capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol 400, N... |
| Active Ingredient | Ethosuximide |
| Dosage Form | Syrup; Capsule |
| Route | Oral |
| Strength | 250mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Teva Pharms; Versapharm; Pharm Assoc; Zydus Pharms Usa; Banner Pharmacaps; Mikart |
| 2 of 4 | |
|---|---|
| Drug Name | Zarontin |
| PubMed Health | Ethosuximide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Zarontin (ethosuximide) is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:Each Zarontin capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol... |
| Active Ingredient | Ethosuximide |
| Dosage Form | Syrup; Capsule |
| Route | Oral |
| Strength | 250mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Parke Davis |
| 3 of 4 | |
|---|---|
| Drug Name | Ethosuximide |
| PubMed Health | Ethosuximide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Ethosuximide is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:Each ethosuximide capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol 400, N... |
| Active Ingredient | Ethosuximide |
| Dosage Form | Syrup; Capsule |
| Route | Oral |
| Strength | 250mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Teva Pharms; Versapharm; Pharm Assoc; Zydus Pharms Usa; Banner Pharmacaps; Mikart |
| 4 of 4 | |
|---|---|
| Drug Name | Zarontin |
| PubMed Health | Ethosuximide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Zarontin (ethosuximide) is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:Each Zarontin capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol... |
| Active Ingredient | Ethosuximide |
| Dosage Form | Syrup; Capsule |
| Route | Oral |
| Strength | 250mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Parke Davis |
Anticonvulsants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ethosuximide, the drug of choice, and phensuximide are indicated for the control of seizures in absence (petit mal) epilepsy. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 271
Hemodialysis patients concurrently receiving ethosuximide may require a supplemental dose or an altered dosing schedule, based on the conclusion that ethosuximide is dialyzable.
MARBURY TC ET AL; AM J HOSP PHARM 38(NOV) 1757 (1981)
The most common dose-related side effects are gastrointestinal complaints (nausea, vomiting, and anorexia) and CNS effects (drowsiness, lethargy, euphoria, dizziness, headache, and hiccough). Some tolerance to these effects develops. Parkinsonlike symptoms and photophobia also have been reported. Restlessness, agitation, anxiety, aggressiveness, inability to concentrate, and other behavioral effects have occurred primarily in patients with a prior history of psychiatric disturbance.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 535
Urticaria and other skin reactions, including Stevens-Johnson syndrome, as well as systemic lupus erythematosus, eosinophilia, leukopenia, thrombocytopenia, pancytopenia, and aplastic anemia also have been attributed to the drug. The leukopenia may be transient, despite continuation of the drug, but several deaths have resulted from bone-marrow depression. Renal or hepatic toxicity has not been reported.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 535
The most common adverse effects of ethosuximide are GI symptoms including anorexia and weight loss, vague gastric upset, cramps, abdominal pain, diarrhea, nausea, vomiting, and epigastric distress.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2218
For more Drug Warnings (Complete) data for ETHOSUXIMIDE (13 total), please visit the HSDB record page.
Estimated fatal dose 5 g /From table/
Dreisbach, R. H. Handbook of Poisoning. 9th ed. Los Altos, California: Lange Medical Publications, 1977., p. 311
For the treatment of petit mal epilepsy.
FDA Label
Treatment of childhood absence epilepsy
Used in the treatment of epilepsy. Ethosuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AD - Succinimide derivatives
N03AD01 - Ethosuximide
Absorption
Bioavailability following oral administration is 93%.
Ethosuximide is absorbed from the GI tract. Following oral administration of a single dose, peak blood concentrations are reached within 4 hours; however, about 4-7 days of therapy at usual dosage are required to achieve steady-state plasma concentrations. The plasma concentration required for therapeutic effect is generally considered to range from 40-100 ug/mL; plasma concentrations less than 40 ug/mL are rarely effective. The relationship between plasma ethosuximide concentrations and toxic effects of the drug has not been clearly established; however, plasma concentrations as high as 150 ug/mL have not been associated with signs of toxicity.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2219
Absorption of ethosuximide appears to be complete, and peak concentrations occur in plasma within about 3 hr after a single oral dose. Ethosuximide is not significantly bound to plasma proteins; during long-term therapy, the concentration in the CSF is similar to that in plasma. The apparent volume of distribution averages 0.7 L/kg.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 535
In vitro data suggest that there is no substantial degree of protein binding for ethosuximide. In one study in children, peak CSF concentrations of 25-50 ug/mL were achieved within 1-2 hours following a single 250-mg dose of ethosuximide. These concentrations were maintained for 12-24 hours, and the drug was still detectable in the CSF 65 hours after the drug was given.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2219
Ethosuximide is excreted slowly in urine. Approximately 20% of a dose is excreted unchanged and up to 50% may be excreted in urine as the hydroxylated metabolite and/or its glucuronide. Small amounts of unchanged drug are also excreted in bile and feces.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2219
For more Absorption, Distribution and Excretion (Complete) data for ETHOSUXIMIDE (9 total), please visit the HSDB record page.
Hepatic, via CYP3A4 and CYP2E1.
... Metabolized by hepatic microsomal enzymes.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 535
In rats, ethosuximide ... is metabolized into monohydroxyethosuximides, 2-ethyl-3-hydroxy-2-methyl-succinimide ... stereoisomeric 2-(1-hydroxyethyl)-2-methylsuccinimides & ... 2-(2-hydrox yethyl)-2-methylsuccinimide ... which are excreted, in urine, in free form and as ether glucuronides.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 262
Different plasma profiles were obtained following admin of ethosuximide...to rat & man... Unchanged drug & only trace amt of metabolites were detected in rat plasma. In human plasma, diastereoisomers of 2-(1-hydroxyethyl)-2-methylsuccinimide...were major components.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 552
Ethosuximide is a chiral drug substance primarily indicated for the treatment of absence seizures. This drug is used clinically as the racemate. The human urinary metabolites of ethosuximide (I) have been studied using chiral gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS). The metabolites identified were the previously reported unchanged ethosuximide (I) enantiomers, all four stereoisomers of 2-(1-hydroxyethyl)-2-methylsuccinimide (II), and the four stereoisomers of 2-ethyl-3-hydroxy-2-methylsuccinimide (III). Through chemical derivatization methodology and GC/MS two enantiomers of a previously unreported metabolite of ethosuximide, 2-ethyl-2-hydroxymethylsuccinimide (VI), have been identified.
PMID:15931663 Millership JS et al; Biopharm Drug Dispos 26 (6): 225-32 (2005)
53 hours
A total of 10 epileptic mothers treated with ethosuximide (ES) as well as their 13 newborns were included in this study. At birth fetal/maternal serum concentration ratios were 0.97 +/- 0.02 (n = 7) and ES half-lives in three neonates were 32, 37 and 38 hr. ...
PMID:6508976 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1463560 Kuhnz W et al; Br J Clin Pharmacol 18 (5): 671-7 (1984)
The plasma half-life of ethosuximide is about 60 hours in adults and about 30 hours in children.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2219
A total of 10 epileptic mothers treated with ethosuximide (ES) as well as their 13 newborns were included in this study. At birth fetal/maternal serum concentration ratios were 0.97 +/- 0.02 (n = 7) and ES half-lives in three neonates were 32, 37 and 38 hr.
PMID:6508976 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1463560 Kuhnz W et al; Br J Clin Pharmacol 18 (5): 671-7 (1984)
Binds to T-type voltage sensitive calcium channels. Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1G gives rise to T-type calcium currents. T-type calcium channels belong to the "low-voltage activated (LVA)" group and are strongly blocked by mibefradil. A particularity of this type of channels is an opening at quite negative potentials and a voltage-dependent inactivation. T-type channels serve pacemaking functions in both central neurons and cardiac nodal cells and support calcium signaling in secretory cells and vascular smooth muscle. They may also be involved in the modulation of firing patterns of neurons which is important for information processing as well as in cell growth processes.
Succinimide anticonvulsants are thought to increase the seizure threshold and suppress the paroxysmal three-cycle-per-second spike-and-wave pattern seen with absence (petit mal) seizures. The frequency of attacks is reduced by depression of nerve transmission in the motor cortex. These effects may be due to direct modification of membrane function in excitable cells and/or alteration of chemically mediated neurotransmission. The specific effect of ethosuximide against absence seizures appears to be due to its ability to block T-type calcium channels at concentrations that do not affect other ion channels. /Succinimide Anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 271
Ethosuximide reduces low threshold Ca(2+) currents (T currents) in thalamic neurons. The thalamus plays an important role in generation of 3 Hz spike-wave rhythms typical of absence seizures. Neurons in the thalamus exhibit a large amplitude T current spike that underlies bursts of action potentials and likely plays an important role in thalamic oscillatory activity such as 3 Hz spike-wave activity. At clinically relevant concentrations, ethosuximide inhibits the T current, as evident in voltage-clamp recordings in acutely isolated, ventrobasal thalamic neurons from rats and guinea pigs. Ethosuximide reduces this current without modifying the voltage dependence of steady-state inactivation or the time course of recovery from inactivation. By contrast, succinimide derivatives with convulsant properties do not inhibit this current. Ethosuximide does not inhibit sustained repetitive firing or enhance GABA responses at clinically relevant concentrations. Current data are consistent with the idea that inhibition of T currents is the mechanism by which ethosuximide inhibits absence seizures.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 535
Ethosuximide is an alternative medicament that is used for coupling of petit mal, especially in childhood. Some clinical observations show that it has secondary effects on the gastro intestinal tract (GIT). The present research tries to define the characteristics of Ethosuximide--the inducted secondary effects on the GIT, and to explain some of the possible mechanisms that cause them. The changes that occur in the GIT of patients cured with Ethosuximide are registered roentgenologically. The main change is the atony of the stomach and intestines and the reduced peristaltic activity. The influence of Ethosuximide is examined on smooth muscular samples of human stomach, taken in vitro using stomach resection. The medicament authoritatively reduce the spontaneous bioelectrical activity of the smooth muscular tissue, influences mainly it's components that have Ca+ nature. Together with that is indicated relaxation of the smooth muscular samples. In that research is expressed the thesis that this Ethosuximide reduction of the Ca(+)-influx in the smooth muscular cells and the related relaxation probably are one of the main reasons of the secondary effects on the GIT.
PMID:10205989 Zagorchev P et al; Folia Med (Plovdiv) 40 (3B Suppl 3): 28-33 (1998)
Ethosuximide is one of the means of treatment of minor epilepsy but hardly any data on its mechanism of action are available in the literature. Anticonvulsant agents are known to bring about changes in the functions and in the interaction between some of the mediator systems within the central nervous system. An assessment of the status of neuromediator systems can be made on the basis of the response of isolated smooth muscle strips to the action of agonists and antagonists of various receptors. It was found by the pharmacological analysis of isolated strips from the rat stomach (antrum and corpus strips), the seminal duct and the cervical vein that ethosuximide induces a reduction in the physical contractile activity and the tone of smooth muscle preparations. Smooth muscle relaxation caused by ethosuximide is not blocked by different receptor inhibitors such as dihydroergotamine, propranolol, atropine, chlorpromazine, haloperidol and indomethacin. Ethosuximide causes a significant reduction in the physical contraction of smooth muscles produced by potassium chloride depolarization, with a stronger impact on the subsequent tonic contraction caused by calcium ions. A reduction in the potassium content of the solution has no effect on the nature of the action of ethosutimide. It is thus assumed that the probable mechanism of action of ethosuximide consists in lowering calcium transport since the inhibitors of calcium transport sodium nitroprusside and verapamil intensify the blocking effect of ethosuximide on smooth muscle contractile activity.
PMID:1860492 Toreva D et al; Farmakol Toksikol 54 (1): 23-7 (1991)