


1. Absele
2. Ethnor
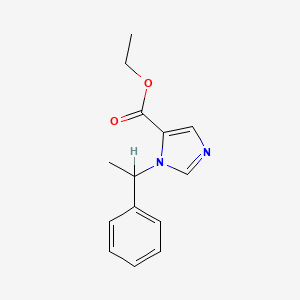
1. 33125-97-2
2. Ethyl 1-(1-phenylethyl)-1h-imidazole-5-carboxylate
3. Ethnor
4. Absele
5. 15301-65-2
6. C14h16n2o2
7. Radenarcon
8. Amidate (pharmaceutical)
9. Mls000034952
10. 1h-imidazole-5-carboxylic Acid, 1-(1-phenylethyl)-, Ethyl Ester, (r)-
11. Smr000010931
12. Dsstox_cid_3033
13. 1h-imidazole-5-carboxylic Acid, 1-(1-phenylethyl)-, Ethyl Ester, (+-)-
14. Dsstox_rid_76841
15. Dsstox_gsid_23033
16. 1-(1-phenylethyl)-1h-imidazole-5-carboxylic Acid Ethyl Ester
17. Ethyl 3-(1-phenylethyl)imidazole-4-carboxylate
18. Sr-01000325781
19. Rac-etomidate
20. Ncgc00016817-01
21. Cas-33125-97-2
22. Cpd000010931
23. Opera_id_1830
24. Prestwick0_001041
25. Prestwick1_001041
26. Prestwick2_001041
27. (+)-ethyl 1-(.alpha.-methylbenzyl)imidazole-5-carboxylate
28. Schembl39485
29. Mls001240191
30. Mls006011948
31. Chembl23731
32. Spbio_002901
33. Chebi:91759
34. Imidazole-5-carboxylic Acid, 1-(.alpha.-methylbenzyl)-, Ethyl Ester, (r)-(+)-
35. Bdbm238149
36. Dtxsid101347652
37. Hms1571a04
38. Hms1612g10
39. Hms2093p17
40. Hms2235k14
41. Hms3259f15
42. Hms3369p19
43. Hms3371k15
44. Hms3393p18
45. Hms3428l14
46. Hms3654n15
47. Pharmakon1600-01505599
48. Bcp21365
49. Us9394290, Eto (etomidate)
50. Tox21_110628
51. Nsc759160
52. S1329
53. Akos000548952
54. Akos021628930
55. Tox21_110628_1
56. Ac-5898
57. Ccg-266910
58. Nc00657
59. Ncgc00025176-02
60. Ncgc00036076-03
61. Ds-14867
62. Sbi-0206822.p001
63. Db-015827
64. Cs-0081287
65. Ft-0630568
66. Sw197275-2
67. En300-50290
68. Ab00439988-11
69. Ab00439988_13
70. Ethyl 3-(1-phenylethyl)imidazole-4-carboxylate.
71. A821630
72. Ethyl1-(1-phenylethyl)-1h-imidazole-5-carboxylate
73. Sr-01000325781-1
74. Sr-01000325781-3
75. Sr-01000325781-5
76. Brd-a54880345-001-11-8
77. Q27163565
78. 3-(1-phenylethyl)-4-imidazolecarboxylic Acid Ethyl Ester
79. Z802671476
80. (-)-ethyl 1-(1-phenylethyl)-1h-imidazole- 5-carboxylate
81. (-)-ethyl 1-(1-phenylethyl)-1h-imidazole-5-carboxylate
82. Ethyl 1-(1-phenylethyl)-1h-imidazole-5-carboxylate, (+)-
83. R-(+) Ethyl 1-(1-phenylethyl)-1h-imidazole-5-carboxylate
84. 1-(.alpha.-methylbenzyl)-1h-imidazole-5-carboxylic Acid Ethyl Ester
85. (r)-(+)-1-(.alpha.-methylbenzyl)imidazole-5-carboxylic Acid Ethyl Ester
86. [r,(+)]-1-(alpha-methylbenzyl)-1h-imidazole-5-carboxylic Acid Ethyl Ester
87. 1h-imidazole-5-carboxylic Acid, 1-(.alpha.-methylbenzyl)-, Ethyl Ester, (+)-
88. R 16659 Pound>>r-16659 Pound>>r16659 Pound>>r 26490 Pound>>r-26490 Pound>>r26490
| Molecular Weight | 244.29 g/mol |
|---|---|
| Molecular Formula | C14H16N2O2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 244.121177757 g/mol |
| Monoisotopic Mass | 244.121177757 g/mol |
| Topological Polar Surface Area | 44.1 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 277 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Amidate |
| Drug Label | Amidate (Etomidate Injection, USP) is a sterile, nonpyrogenic solution. Each milliliter contains etomidate, 2 mg, propylene glycol 35% v/v. The pH is 6.0 (4.0 to 7.0).It is intended for the induction of general anesthesia by intravenous injection.The... |
| Active Ingredient | Etomidate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 4 | |
|---|---|
| Drug Name | Etomidate |
| Drug Label | Etomidate Injection, USP is a sterile, nonpyrogenic solution. Each milliliter contains etomidate, 2 mg, propylene glycol 35% v/v. The pH is 6.0 (4.0 to 7.0).It is intended for the induction of general anesthesia by intravenous injection.The drug etom... |
| Active Ingredient | Etomidate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Par Sterile Products; Bedford; Emcure Pharms; Luitpold; Zydus Pharms Usa; Agila Speclts |
| 3 of 4 | |
|---|---|
| Drug Name | Amidate |
| Drug Label | Amidate (Etomidate Injection, USP) is a sterile, nonpyrogenic solution. Each milliliter contains etomidate, 2 mg, propylene glycol 35% v/v. The pH is 6.0 (4.0 to 7.0).It is intended for the induction of general anesthesia by intravenous injection.The... |
| Active Ingredient | Etomidate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Hospira |
| 4 of 4 | |
|---|---|
| Drug Name | Etomidate |
| Drug Label | Etomidate Injection, USP is a sterile, nonpyrogenic solution. Each milliliter contains etomidate, 2 mg, propylene glycol 35% v/v. The pH is 6.0 (4.0 to 7.0).It is intended for the induction of general anesthesia by intravenous injection.The drug etom... |
| Active Ingredient | Etomidate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml |
| Market Status | Prescription |
| Company | Par Sterile Products; Bedford; Emcure Pharms; Luitpold; Zydus Pharms Usa; Agila Speclts |
N01AX07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AX - Other general anesthetics
N01AX07 - Etomidate