

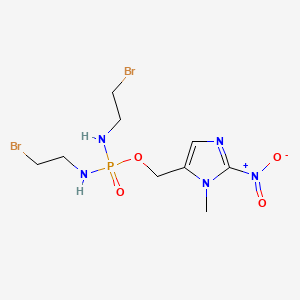

1. Th 302
2. Th-302
3. Th302 Cpd
1. Th-302
2. 918633-87-1
3. Th302
4. Th 302
5. Hap-302
6. N,n'-bis(2-bromoethyl)phosphorodiamidic Acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl Ester
7. 2-bromo-n-[(2-bromoethylamino)-[(3-methyl-2-nitroimidazol-4-yl)methoxy]phosphoryl]ethanamine
8. 8a9rz3hn8w
9. Compound 3b
10. Th-302; Evofosfamide;hap-302
11. Phosphorodiamidic Acid, N,n'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1h-imidazol-5-yl)methyl Ester
12. Phosphorodiamidic Acid, N,n'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1h-imidazol-5-yl)methyl Ester.
13. Evofosfamide [usan:inn]
14. Unii-8a9rz3hn8w
15. Evofosfamide(th 302)
16. Evofosfamide(th-302)
17. Evofosfamide [inn]
18. Evofosfamide [jan]
19. Evofosfamide [usan]
20. Evofosfamide [who-dd]
21. Chembl260046
22. Evofosfamide (jan/usan/inn)
23. Gtpl8695
24. Schembl2357174
25. Schembl15691894
26. Dtxsid60238721
27. Amy16615
28. Bcp06074
29. Ex-a1740
30. Bdbm50543112
31. S2757
32. Zinc29053729
33. Akos026673946
34. Ccg-269225
35. Cs-0616
36. Db06091
37. Sb17116
38. Ac-29025
39. As-74462
40. Hy-10535
41. Ft-0696771
42. J3.504.735b
43. D10704
44. A901406
45. J-523214
46. Q7670203
47. (1-methyl-2-nitro-1h-imidazol-5-yl)methyl N,n'-bis(2-bromoethyl)diamidophosphate
48. (1-methyl-2-nitro-1h-imidazole-5-yl)methyl N,n'-bis(2-bromoethyl) Diamidophosphate
49. 1-methyl-2-nitro-l H-imidazole-5-yl) N,n-bis(2-bromoethyl)diamidophosphate
50. (2-bromoethyl)({[(2-bromoethyl)amino][(1-methyl-2-nitro-1h-imidazol-5-yl)methoxy]phosphoryl})amine
51. Th302n,n'-bis(2-bromoethyl)phosphorodiamidic Acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl Ester
| Molecular Weight | 449.04 g/mol |
|---|---|
| Molecular Formula | C9H16Br2N5O4P |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 448.92862 g/mol |
| Monoisotopic Mass | 446.93067 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 374 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in solid tumors.
TH-302 combines a 2-nitroimidazole oxygen-sensing trigger with a masked DNA crosslinker. Upon activation in oxygen deficient zones, TH-302 is converted selectively to the drug's active form, dibromo isophosphoramide mustard, a potent alkylator. TH-302 targets levels of severe hypoxia that are found in tumors but are rare in normal tissues - this is how selective targeting of the tumor occurs. After conversion to the active form of the drug, the hypoxic cells are exposed to high concentrations of released cytotoxic agent, which can also diffuse into the adjacent regions of the tumor.