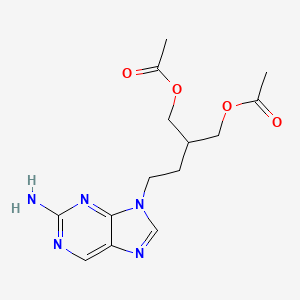

1. 1,3-propanediol, 2-(2-(2-amino-9h-purin-9-yl)ethyl)-, Diacetate (ester)
2. 9-(4-acetoxy-3-(acetoxymethyl)but-1-yl)-2-aminopurine
3. Brl 42810
4. Brl-42810
5. Brl42810
6. Famvir
1. 104227-87-4
2. Famvir
3. Brl-42810
4. Famciclovirum
5. 2-(2-(2-amino-9h-purin-9-yl)ethyl)propane-1,3-diyl Diacetate
6. Famciclovirum [inn-latin]
7. Brl 42810
8. [2-(acetyloxymethyl)-4-(2-aminopurin-9-yl)butyl] Acetate
9. Famciclovir (famvir)
10. 2-[(acetyloxy)methyl]-4-(2-amino-9h-purin-9-yl)butyl Acetate
11. Fcv
12. Chebi:4974
13. 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine
14. Nsc-758921
15. Qic03ani02
16. 1,3-propanediol, 2-(2-(2-amino-9h-purin-9-yl)ethyl)-, Diacetate (ester)
17. 2-(2-(2-amino-9h-purin-9-yl)ethyl)-1,3-propanediol Diacetate (ester)
18. Ncgc00095133-01
19. Famciclovir 100 Microg/ml In Acetonitrile
20. 2-(2-(2-amino-9h-purin-9-yl)ethyl)-1,3-propanediol Diacetate
21. 2-[2-(2-amino-9h-purin-9-yl)ethyl]-1,3-propanediol Diacetate
22. Dsstox_cid_3038
23. Dsstox_rid_76845
24. Dsstox_gsid_23038
25. Oravir
26. Acetic Acid 2-acetoxymethyl-4-(2-amino-purin-9-yl)-butyl Ester
27. 2-(acetoxymethyl)-4-(2-amino-4,5-dihydro-9h-purin-9-yl)butyl Acetate
28. 2-[2-(2-amino-9h-purin-9-yl)ethyl]trimethylene Diacetate;2-[2-(2-amino-9h-purin-9-yl)ethyl]trimethylene Diacetate
29. Smr000466375
30. Famvir (tn)
31. Cas-104227-87-4
32. Sr-01000759358
33. Unii-qic03ani02
34. Famciclovir [usan:inn:ban]
35. 1,3-propanediol, 2-[2-(2-amino-9h-purin-9-yl)ethyl]-, Diacetate (ester)
36. 2-[2-(2-amino-9h-purin-9-yl)ethyl]-1,3-propanediol Diacetate (ester)
37. Hsdb 8121
38. Famciclovir,(s)
39. Brl42810
40. Famciclovir, Bio-x
41. Mfcd00866964
42. 1,3-diyl Diacetate
43. Spectrum_000466
44. Famciclovir [mi]
45. Spectrum2_001101
46. Spectrum3_001675
47. Spectrum4_000611
48. Spectrum5_001548
49. Famciclovir [inn]
50. Famciclovir [jan]
51. Famciclovir [usan]
52. Chembl880
53. Famciclovir [vandf]
54. Schembl2949
55. Famciclovir [mart.]
56. Bspbio_003489
57. Famciclovir [usp-rs]
58. Famciclovir [who-dd]
59. Kbiogr_001162
60. Kbioss_000946
61. Mls000759505
62. Mls001424115
63. Mls006011896
64. Famciclovir (jan/usp/inn)
65. Spectrum1505201
66. Spbio_001202
67. Dtxsid0023038
68. Famciclovir, >=98% (hplc)
69. Gtpl11629
70. Kbio2_000946
71. Kbio2_003514
72. Kbio2_006082
73. Kbio3_002709
74. [2-(acetoxymethyl)-4-(2-aminopurin-9-yl)butyl] Acetate
75. Famciclovir [orange Book]
76. Hms1922l09
77. Hms2051g22
78. Hms2090g10
79. Hms2093k12
80. Hms2232h09
81. Hms3262o08
82. Hms3370d14
83. Hms3393g22
84. Hms3656a04
85. Hms3715f03
86. Pharmakon1600-01505201
87. Famciclovir [usp Monograph]
88. Albb-027262
89. Bcp21312
90. Diacetyl 6-deoxy-9-(4-hydroxy-3-hydroxymethyl-but-1-yl)guanine
91. Zinc1530635
92. Tox21_111442
93. Tox21_500793
94. Ak-120
95. Bdbm50248001
96. Ccg-39277
97. Nsc758921
98. S2467
99. Stk623204
100. 2-(2-(2-amino-9h-purin-9-yl)ethyl)-1,3-propanediol Diacetate Ester
101. Akos005556609
102. Tox21_111442_1
103. Ab07547
104. Ac-8066
105. Cs-1357
106. Db00426
107. Ks-5020
108. Nc00156
109. Nsc 758921
110. Ncgc00095133-02
111. Ncgc00095133-03
112. Ncgc00095133-04
113. Ncgc00095133-06
114. Ncgc00095133-15
115. Ncgc00261478-01
116. Bf164441
117. Hy-17426
118. Sbi-0206743.p001
119. Db-014809
120. F0842
121. Ft-0602549
122. Ft-0668461
123. Sw197536-3
124. A19583
125. D00317
126. 2-(2-(2-amino-9h-purin-9-yl)ethyl)propane-
127. Ab00639952-06
128. Ab00639952-08
129. Ab00639952_09
130. Ab00639952_10
131. 227f874
132. Q420186
133. Brl-42810;brl42810;brl 42810;famciclovirum
134. J-001134
135. Sr-01000759358-4
136. Sr-01000759358-5
137. 9-(4-acetoxy-3-acetoxymethylbut-1-yl)-2-aminopurine
138. Brd-k45033733-001-02-3
139. Brd-k45033733-001-05-6
140. 9-[4-acetoxy-3-(acetoxymethyl)butyl]-2-amino-9h Purine
141. [2-(acetyloxymethyl)-4-(2-aminopurin-9-yl)-butyl] Acetate
142. 2-(2-(2-amino-9h-purin-9-yl)ethyl)-propane-1,3-diyl Diacetate
143. Famciclovir, United States Pharmacopeia (usp) Reference Standard
144. Famciclovir, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 321.33 g/mol |
|---|---|
| Molecular Formula | C14H19N5O4 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 321.14370410 g/mol |
| Monoisotopic Mass | 321.14370410 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 404 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Famciclovir |
| PubMed Health | Famciclovir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | Famciclovir tablets contains famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate. It is a synthetic acyclic guanine derivativ... |
| Active Ingredient | Famciclovir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg; 500mg |
| Market Status | Prescription |
| Company | Hetero Labs Ltd V; Teva Pharms; Macleods Pharms; Apotex; Aurobindo Pharma; Cipla; Mylan; Roxane |
| 2 of 4 | |
|---|---|
| Drug Name | Famvir |
| PubMed Health | Famciclovir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | The active ingredient in FAMVIR tablets is famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate. Its molecular formula is C14H... |
| Active Ingredient | Famciclovir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg; 500mg |
| Market Status | Prescription |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Famciclovir |
| PubMed Health | Famciclovir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | Famciclovir tablets contains famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate. It is a synthetic acyclic guanine derivativ... |
| Active Ingredient | Famciclovir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg; 500mg |
| Market Status | Prescription |
| Company | Hetero Labs Ltd V; Teva Pharms; Macleods Pharms; Apotex; Aurobindo Pharma; Cipla; Mylan; Roxane |
| 4 of 4 | |
|---|---|
| Drug Name | Famvir |
| PubMed Health | Famciclovir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | The active ingredient in FAMVIR tablets is famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate. Its molecular formula is C14H... |
| Active Ingredient | Famciclovir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg; 500mg |
| Market Status | Prescription |
| Company | Novartis |
Antiviral Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2013)
Oral famciclovir is used for the treatment of acute, localized herpes zoster (shingles, zoster). /Included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 812
Oral famciclovir is used for the treatment of recurrent mucocutaneous herpes simplex virus (HSV) infections (HSV-1 and HSV-2) in HIV-infected adults. /Included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 813
Famciclovir has been used for the management of chronic hepatitis B virus (HBV) infection in a limited number of patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 813
For more Therapeutic Uses (Complete) data for Famciclovir (12 total), please visit the HSDB record page.
/These/ adverse events have been reported during post-approval use of Famvir. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Blood and lymphatic system disorders: Thrombocytopenia. Hepatobiliary disorders: Abnormal liver function tests, cholestatic jaundice. Nervous system disorders: Dizziness, somnolence. Psychiatric disorders: Confusion (including delirium, disorientation, and confusional state occurring predominantly in the elderly), hallucinations. Skin and subcutaneous tissue disorders: Urticaria, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, angioedema (e.g. face, eyelid, periorbital, and pharyngeal edema).
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348
The manufacturer recommends that the dosage interval of famciclovir be adjusted carefully in patients with impaired renal function to prevent drug accumulation while maintaining adequate plasma concentrations of penciclovir, the active metabolite of famciclovir.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 815
Increased serum concentrations of ALT (SGPT) occurred in 1.4-2.4% of patients receiving famciclovir in clinical trials for herpes zoster or genital herpes. Increased serum concentrations of alkaline phosphatase, total bilirubin, and albumin each occurred rarely in patients receiving the drug in clinical trials for herpes zoster or genital herpes.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 814
The most frequent adverse GI effect of famciclovir is nausea which occurred in approximately 13% of patients receiving the drug (versus in 11.6% in placebo recipients) in a large, controlled clinical trial for herpes zoster. Nausea resulted in discontinuance of famciclovir in less than 1% of patients in clinical trials for herpes zoster or genital herpes. Diarrhea was reported in approximately 8% of patients (5% of placebo recipients) and vomiting in approximately 5% of patients (3.4% of placebo recipients) in a large, controlled clinical trial for herpes zoster. Vomiting only rarely resulted in discontinuance of famciclovir in clinical trials for herpes zoster or genital herpes. Constipation, anorexia, abdominal pain, flatulence, and dyspepsia have occurred in patients receiving famciclovir in clinical trials for herpes zoster. Acute necroticohemorrhagic pancreatitis resulting in death has been reported following famciclovir administration for severe hepatitis B virus infection in a kidney graft recipient who was receiving cyclosporine concomitantly; a causal relationship to famciclovir was not established. Nausea, diarrhea, vomiting, or abdominal pain has been reported in 11, 7, 5, or 3%, respectively, of HIV-infected patients receiving famciclovir in clinical studies.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 814
For more Drug Warnings (Complete) data for Famciclovir (12 total), please visit the HSDB record page.
For the treatment of acute herpes zoster (shingles). Also for the treatment or suppression of recurrent genital herpes in immunocompetent patients and treatment of recurrent mucocutaneous herpes simplex infections in HIV infected patients.
FDA Label
Famciclovir is a prodrug that undergoes rapid biotransformation to the active antiviral compound penciclovir. Penciclovir is an anti-viral drug which has inhibitory activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and varicella zoster virus (VZV). Therefore, herpes viral DNA synthesis and replication are selectively inhibited.
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB09 - Famciclovir
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AD - Antivirals
S01AD07 - Famciclovir
Absorption
77 %
Route of Elimination
Active tubular secretion contributes to the renal elimination of penciclovir.
Volume of Distribution
1.080.17 L/kg [healthy male subjects following a single intravenous dose of penciclovir at 400 mg administered as a 1-hour intravenous infusion]
Clearance
36.6 +/- 6.3 L/hr [healthy male]
0.48 +/- 0.09 L/hr/kg [healthy male]
Following oral administration of famciclovir to lactating rats, penciclovir was distributed into breast milk at concentrations higher than those observed in plasma.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 815
Not known whether penciclovir crosses the placenta or is distributed into human milk.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 815
Following oral single-dose administration of 500 mg famciclovir to seven patients with herpes zoster, the AUC (mean + or - SD), Cmax, and tmax were 12.1+ or - 1.7 ug hr/mL, 4.0 + or - 0.7 ug/mL, and 0.7 + or - 0.2 hours, respectively. The AUC of penciclovir was approximately 35% greater in patients with herpes zoster as compared to healthy volunteers. Some of this difference may be due to differences in renal function between the two groups.
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348
Renal clearance of penciclovir following the oral administration of a single 500 mg dose of famciclovir to 109 healthy male volunteers was 27.7+ or - 7.6 L/hr. Active tubular secretion contributes to the renal elimination of penciclovir.
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348
For more Absorption, Distribution and Excretion (Complete) data for Famciclovir (11 total), please visit the HSDB record page.
Hepatic
Famciclovir is deacetylated and oxidized to penciclovir. Penciclovir is phosphorylated to penciclovir triphosphate (the active metabolite) in cells infected with HSV-1, HSV-2, or VZV. The inactive metabolite 6-deoxy penciclovir is converted to penciclovir by aldehyde oxidase. Famciclovir not metabolized by CYP enzymes.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 815
Following oral administration, famciclovir is deacetylated and oxidized to form penciclovir. Metabolites that are inactive include 6-deoxy penciclovir, monoacetylated penciclovir, and 6-deoxy monoacetylated penciclovir (5%, <0.5% and <0.5% of the dose in the urine, respectively). Little or no famciclovir is detected in plasma or urine. An in vitro study using human liver microsomes demonstrated that cytochrome P450 does not play an important role in famciclovir metabolism. The conversion of 6-deoxy penciclovir to penciclovir is catalyzed by aldehyde oxidase.
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348
10 hours
Elimination half-life of penciclovir after oral administration of famciclovir 1.6-3 hours. Intracellular half-life of penciclovir triphosphate in cells infected with herpes simplex virus (HSV)-1 or HSV-2 is 10 and 20 hours, respectively; intracellular half-life in varicella zoster virus (VZV)-infected cells is 7-14 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 815
The plasma elimination half-life of penciclovir was 2.0 + or - 0.3 hours after intravenous administration of penciclovir to 48 healthy male volunteers and 2.3 + or - 0.4 hours after oral administration of 500 mg famciclovir to 124 healthy male volunteers. The half-life in 17 patients with herpes zoster was 2.8 + or - 1.0 hours and 2.7 + or - 1.0 hours after single and repeated doses, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348
Famciclovir undergoes rapid biotransformation to the active antiviral compound penciclovir, which has inhibitory activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and varicella zoster virus (VZV). In cells infected with HSV-1, HSV-2 or VZV, viral thymidine kinase phosphorylates penciclovir to a monophosphate form that, in turn, is converted to penciclovir triphosphate by cellular kinases. In vitro studies demonstrate that penciclovir triphosphate inhibits HSV-2 DNA polymerase competitively with deoxyguanosine triphosphate. Consequently, herpes viral DNA synthesis and, therefore, replication are selectively inhibited.
Famciclovir is a prodrug of penciclovir, which has demonstrated inhibitory activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and varicella zoster virus (VZV). In cells infected with HSV-1, HSV-2 or VZV, the viral thymidine kinase phosphorylates penciclovir to a monophosphate form that, in turn, is converted by cellular kinases to the active form penciclovir triphosphate. Biochemical studies demonstrate that penciclovir triphosphate inhibits HSV-2 DNA polymerase competitively with deoxyguanosine triphosphate. Consequently, herpes viral DNA synthesis and, therefore, replication are selectively inhibited. Penciclovir triphosphate has an intracellular half-life of 10 hours in HSV-1-, 20 hours in HSV-2- and 7 hours in VZV-infected cells grown in culture. However, the clinical significance of the intracellular half-life is unknown.
US Natl Inst Health; DailyMed. Current Medication Information for FAMVIR (famciclovir) tablet, film coated (February 2012). Available from, as of March 16, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3c6194c0-ba69-4b16-b882-caf736a02348