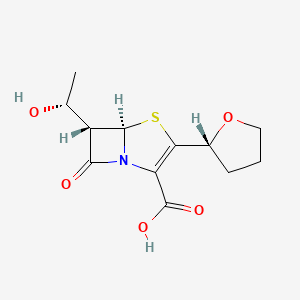

1. (5r,6s)-6-(r)-1-hydroxyethyl-7-oxo-3-(r)-2-tetrahydrofuryl-4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylate
2. Fropenem
1. Fropenem
2. 106560-14-9
3. Faropenem Sodium
4. Faropenem Sodium Hydrate
5. (5r,6s)-6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-oxolan-2-yl]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
6. (5r,6s)-6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-tetrahydrofuran-2-yl]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
7. Chebi:51257
8. F52y83bgh3
9. (+)-(5r,6s)-6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydro-2-furyl)-4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid
10. 122547-49-3
11. Ncgc00164546-02
12. 122547-49-3 (na)
13. Dsstox_cid_26430
14. Dsstox_rid_81608
15. Dsstox_gsid_46430
16. (5r,6s)-6-((r)-1-hydroxyethyl)-7-oxo-3-((r)-tetrahydrofuran-2-yl)-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
17. 6alpha-[(1r)-1-hydroxyethyl]-2-[(2r)-tetrahydrofuran-2-yl]-2,3-didehydropenam-3-carboxylic Acid
18. 6alpha-[(r)-1-hydroxyethyl]-2-[(r)-tetrahydrofuran-2-yl]pen-2-em-3-carboxylic Acid
19. Fropenem [inn]
20. (5r,6s)-6-(1-hydroxyethyl)-7-oxo-3-[(2r)-oxolan-2-yl]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
21. Cas-106560-14-9
22. Faropenem [usan:inn]
23. Unii-f52y83bgh3
24. Ncgc00164546-01
25. (5r,6s)-6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydrofuran-2-yl)-4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid
26. Faropenem [inn]
27. Faropenem [mi]
28. Faropenem [who-dd]
29. Schembl239381
30. Chembl556262
31. Dtxsid0046430
32. Gtpl10808
33. Hy-a0035
34. Zinc3959242
35. Tox21_112175
36. Bdbm50483324
37. Compound 1 [pmid: 9216830]
38. Akos015895072
39. Tox21_112175_1
40. Db12190
41. Cs-0006739
42. H11961
43. 560f149
44. A801462
45. Sr-01000872591
46. J-001609
47. Q1397045
48. Sr-01000872591-1
49. 4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydro-2-furanyl)-,(5r,6s)
50. 4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid,6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-tetrahydro-2-furanyl]-, (5r,6s)-
| Molecular Weight | 285.32 g/mol |
|---|---|
| Molecular Formula | C12H15NO5S |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 285.06709375 g/mol |
| Monoisotopic Mass | 285.06709375 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 477 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DI - Other cephalosporins and penems
J01DI03 - Faropenem