

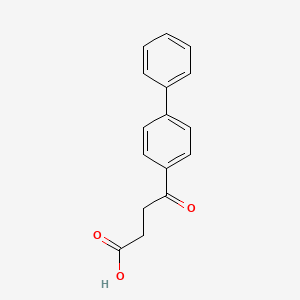

1. Cinopal
2. Gamma-oxo(1,1'-biphenyl)-4-butanoic Acid
3. Lederfen
1. 36330-85-5
2. Lederfen
3. Cinopal
4. Bufemid
5. Napanol
6. 4-(4-biphenylyl)-4-oxobutyric Acid
7. 3-(4-phenylbenzoyl)propionic Acid
8. 3-(4-biphenylylcarbonyl)propionic Acid
9. 4-([1,1'-biphenyl]-4-yl)-4-oxobutanoic Acid
10. 4-(biphenyl-4-yl)-4-oxobutanoic Acid
11. Cl-82204
12. Gamma-oxo(1,1'-biphenyl)-4-butanoic Acid
13. 3-(4-biphenylcarbonyl)propionic Acid
14. 4-oxo-4-(4-phenylphenyl)butanoic Acid
15. Cl 82204
16. 4-(4-biphenyl)-4-oxobutyric Acid
17. 4-biphenyl-4-yl-4-oxobutanoic Acid
18. Butyric Acid 4-(4-biphenyl)-4-oxo-
19. Mls000069810
20. Propionic Acid, 3-(4-biphenylylcarbonyl)-
21. Mfcd00056701
22. Nsc-757812
23. Smr000059150
24. Cl 82,204
25. Chembl277522
26. Chebi:31599
27. 9815r1wr9b
28. [1,1'-biphenyl]-4-butanoic Acid, .gamma.-oxo-
29. Fenbufen 100 Microg/ml In Acetonitrile
30. Ncgc00016834-01
31. Cas-36330-85-5
32. Dsstox_cid_3043
33. 4-[1,1'-biphenyl-4-yl]-4-oxobutanoic Acid
34. Dsstox_rid_76849
35. Dsstox_gsid_23043
36. Fenbufenum
37. Cinopol
38. Fenbufene
39. Fenbufene [inn-french]
40. Fenbufenum [inn-latin]
41. Beta,p-phenylbenzoylpropionic Acid
42. 4-{[1,1'-biphenyl]-4-yl}-4-oxobutanoic Acid
43. Sr-01000721906
44. Einecs 252-979-0
45. Brn 2378560
46. Diphenyl-4-gamma-oxo-gamma-butyric Acid
47. (1,1'-biphenyl)-4-butanoic Acid, .gamma.-oxo-
48. Unii-9815r1wr9b
49. Fenbufen [usan:inn:ban:jan]
50. Fenbufen,(s)
51. Prestwick_567
52. (1,1'-biphenyl)-4-butanoic Acid, Gamma-oxo-
53. Fenbufen, 96%
54. Spectrum_001248
55. Fenbufen [usan]
56. Opera_id_464
57. Fenbufen [inn]
58. Fenbufen [jan]
59. Fenbufen [mi]
60. Prestwick0_000218
61. Prestwick1_000218
62. Prestwick2_000218
63. Prestwick3_000218
64. Spectrum2_001389
65. Spectrum3_001430
66. Spectrum4_000411
67. Spectrum5_001528
68. Fenbufen [mart.]
69. Fenbufen [who-dd]
70. Schembl25117
71. Bspbio_000235
72. Bspbio_003140
73. Fenbufen, Analytical Standard
74. Kbiogr_000702
75. Kbioss_001728
76. 3-10-00-03334 (beilstein Handbook Reference)
77. Mls001074090
78. Divk1c_000025
79. Spectrum1501008
80. Spbio_001378
81. Spbio_002156
82. Bpbio1_000259
83. Fenbufen (jp17/usan/inn)
84. Zinc1427
85. Fenbufen [ep Monograph]
86. Dtxsid9023043
87. Hms500b07
88. Kbio1_000025
89. Kbio2_001728
90. Kbio2_004296
91. Kbio2_006864
92. Kbio3_002360
93. Ninds_000025
94. Hms1568l17
95. Hms1921b13
96. Hms2090g14
97. Hms2092b03
98. Hms2095l17
99. Hms2235i24
100. Hms3372m18
101. Hms3712l17
102. Hms3885n06
103. Pharmakon1600-01501008
104. 4-biphenyl-4-yl-4-oxobutyric Acid
105. Bcp12106
106. Hy-b1138
107. .beta.,p-phenylbenzoylpropionic Acid
108. 3-(4-phenyl)benzoyl Propionic Acid
109. Tox21_110638
110. Bdbm50240374
111. Ccg-38988
112. Nsc757812
113. S4526
114. Stk202178
115. 4-biphenyl-4-yl-4-oxo-butyric Acid
116. Akos000200430
117. Tox21_110638_1
118. Cs-4743
119. Db08981
120. Nsc 757812
121. Ss-4225
122. 3-(4'-biphenylcarbonyl) Propanoic Acid
123. Idi1_000025
124. 3-(4-biphenylylcarbonyl) Propionic Acid
125. 3-(4-biphenylylcarbonyl)-propionic Acid
126. Ncgc00016834-02
127. Ncgc00016834-03
128. Ncgc00016834-04
129. Ncgc00016834-07
130. Ncgc00094886-01
131. Ncgc00094886-02
132. Ncgc00094886-03
133. Ac-14459
134. Sbi-0051631.p002
135. Ab00052195
136. Bb 0221124
137. Ft-0626394
138. Gamma-oxo(1,1''-biphenyl)-4-butanoic Acid
139. .gamma.-oxo(1,1'-biphenyl)-4-butanoic Acid
140. Diphenyl-4-.gamma.-oxo-.gamma.-butyric Acid
141. 4-([1,1'-biphenyl]-4-yl)-4-oxobutanoicacid
142. 4-[1,1''-biphenyl-4-yl]-4-oxobutanoic Acid
143. D01344
144. 4-[1,1'-biphenyl]-4-yl-4-oxobutanoic Acid #
145. Ab00052195-13
146. Ab00052195_14
147. Q2304195
148. Sr-01000721906-2
149. Sr-01000721906-4
150. Sr-01000721906-5
151. Brd-k12513978-001-05-0
152. Brd-k12513978-001-16-7
153. Z99599540
154. Fenbufen, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 254.28 g/mol |
|---|---|
| Molecular Formula | C16H14O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 254.094294304 g/mol |
| Monoisotopic Mass | 254.094294304 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE05 - Fenbufen

LOOKING FOR A SUPPLIER?