


1. Ferinject
2. Injectafer
3. Iron Carboxymaltose
4. Iron Dextri-maltose
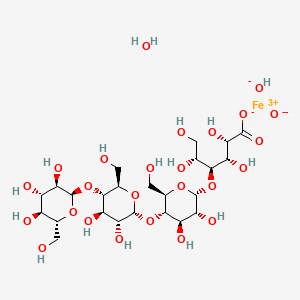
5. Polynuclear Iron (iii)-hydroxide 4(r)-(poly-(1->4)-o-alpha-d-glucopyranosyl)-oxy-2(r),3(s),5(r), 6-tetrahydroxy-hexanoate
6. Vit 45
7. Vit-45
1. 9007-72-1
2. Ex-a3450
3. (2s,3s,4s,5r)-4-[(2r,3r,4r,5s,6r)-5-[(2r,3r,4r,5s,6r)-3,4-dihydroxy-6-(hydroxymethyl)-5-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2,3,5,6-tetrahydroxyhexanoate;iron(3+);oxygen(2-);hydroxide;hydrate
| Molecular Weight | 788.4 g/mol |
|---|---|
| Molecular Formula | C24H44FeO25- |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 25 |
| Rotatable Bond Count | 13 |
| Exact Mass | 788.152102 g/mol |
| Monoisotopic Mass | 788.152102 g/mol |
| Topological Polar Surface Area | 382 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | -1 |
| Complexity | 940 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
| 1 of 2 | |
|---|---|
| Drug Name | Injectafer |
| PubMed Health | ferric carboxymaltose |
| Drug Classes | Iron Supplement |
| Drug Label | Ferric carboxymaltose, an iron replacement product, is an iron carbohydrate complex with the chemical name of polynuclear iron (III) hydroxide 4(R)-(poly-(14)-O--D-glucopyranosyl)-oxy-2(R),3(S),5(R),6-tetrahydroxy-hexanoate. It has a relative mo... |
| Active Ingredient | Ferric carboxymaltose |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 750mg iron/15ml (50mg iron/ml) |
| Market Status | Prescription |
| Company | Luitpold |
| Patent | 7612109; 7754702 |
| 2 of 2 | |
|---|---|
| Drug Name | Injectafer |
| PubMed Health | ferric carboxymaltose |
| Drug Classes | Iron Supplement |
| Drug Label | Ferric carboxymaltose, an iron replacement product, is an iron carbohydrate complex with the chemical name of polynuclear iron (III) hydroxide 4(R)-(poly-(14)-O--D-glucopyranosyl)-oxy-2(R),3(S),5(R),6-tetrahydroxy-hexanoate. It has a relative mo... |
| Active Ingredient | Ferric carboxymaltose |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 750mg iron/15ml (50mg iron/ml) |
| Market Status | Prescription |
| Company | Luitpold |
| Patent | 7612109; 7754702 |
Ferric carboxymaltose is a iron replacement product indicated for the treatment of iron deficiency anemia in adult patients who have intolerance to oral iron or have had unsatisfactory response to oral iron or those who have non-dialysis dependent chronic kidney disease.
FDA Label
When measured using positron emission tomography (PET), the red cell uptake of 59-Fe and 52-Fe from INJECTAFER ranged from 61% to 99%. In patients with iron deficiency, the red cell uptake ranged from 91% to 99%. In patients with renal anemia, the red cell uptake ranged from 61% to 84%.
Absorption
When a single dose of 100 to 1000 mg of iron was given to iron deficient patients, the maximum serum concentration (Cmax) was 37 g/mL to 333 g/mL. These levels were obtained 15 minutes to 1.21 hours post dose (Tmax).
Route of Elimination
Renal elimination of iron was negligible.
Volume of Distribution
3 L
7 to 12 hours.
Ferric carboxymaltose is a colloidal iron (III) hydroxide in complex with carboxymaltose, a carbohydrate polymer that release iron.
