


1. Fesoterodine Fumarate
2. Toviaz
1. 286930-02-7
2. (r) Fesoterodine
3. Fesoterodine (inn)
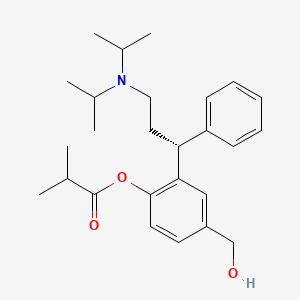
4. [2-[(1r)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate
5. Fesoterodine [inn]
6. (r)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl Isobutyrate
7. 621g617227
8. Propanoic Acid, 2-methyl-, 2-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl Ester
9. Fesoterodine [inn:ban]
10. [2-[(1r)-3-(di(propan-2-yl)amino)-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate
11. Unii-621g617227
12. Feso
13. Starbld0000599
14. Fesoterodine [mi]
15. Fesoterodine [vandf]
16. Fesoterodine [mart.]
17. Fesoterodine [who-dd]
18. Schembl121127
19. Gtpl7473
20. Chembl1201764
21. Dtxsid80182853
22. Chebi:135920
23. Cs-m2392
24. Zinc1552908
25. Akos015841710
26. Db06702
27. Ncgc00346540-01
28. Ncgc00346540-02
29. Ncgc00346540-03
30. Ac-32493
31. Hy-70053
32. D07226
33. Ab01274866-01
34. Ab01274866_02
35. 930f027
36. Q4482372
37. 2-[(1r)-3-[bis(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl 2-methylpropanoate
| Molecular Weight | 411.6 g/mol |
|---|---|
| Molecular Formula | C26H37NO3 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 11 |
| Exact Mass | 411.27734404 g/mol |
| Monoisotopic Mass | 411.27734404 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 491 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | TOVIAZ |
| Active Ingredient | FESOTERODINE FUMARATE |
| Company | PFIZER (Application Number: N022030. Patents: 6858650, 7384980, 7807715, 7855230, 7985772, 8088398, 8338478, 8501723) |
For the treatment of overactive bladder (with symptoms of urinary frequency, urgency, or urge incontinence).
FDA Label
Treatment of the symptoms (increased urinary frequency and / or urgency and / or urgency incontinence) that may occur in patients with overactive-bladder syndrome.
In-vivo the fesoteridine prodrug is broken down into its active metabolite, 5-hydroxymethyl tolterodine (5-HMT), by plasma esterases. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors. Therefore, acting as a competitive muscarinic receptor antagonist, fesoterodine ultimately acts to decrease the detrusor pressure by its muscarinic antagonism, thereby decreasing bladder contraction and consequently, the urge to urinate.
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04BD11
G04BD11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BD - Drugs for urinary frequency and incontinence
G04BD11 - Fesoterodine
Absorption
Tmax (5-HMT): 5 hours post-adminitration of fesoterodine. AUC (0,)= 49.5 ngh/ ml Bioavailability, 5-HMT = 52%
Route of Elimination
Renal: 70% of fesoterodine was recovered in urine as 5-HMT; 35% carboxy metabolite; 18% carboxy-N-desisopropylmetabolite, and 1% N-desisopropyl metabolite Fecal: 7% Hepatic: fesoterodine elimination via CYP2D6 and CYP3A4
Volume of Distribution
IV, 5-HMT: 169 L
Clearance
5-HMT, healthy subjects: 14.4 L/h 5-HMT is also secreted into the nephron.
Metabolized by ubiquitous, nonspecific esterases to transform fesoterodine into 5-HMT Extensive metabolism via CYP2D6 and CYP3A4 into inactive metabolites
7-8 hours for the active metabolite 5-hydroxymethyl tolterodine
Fesoterodine, once converted to its active metabolite, 5-hydroxymethyltolterodine, acts as a competitive antagonists at muscarinic receptors. This results in the inhibition of bladder contraction, decrease in detrusor pressure, and an incomplete emptying of the bladder.