

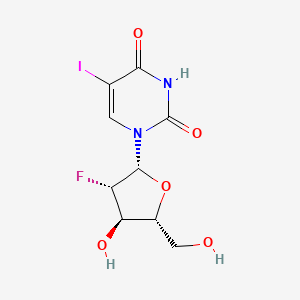

1. 1-(2'fluoro-2'-deoxyarabinofuranosyl)-5-iodouracil
2. 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodo-2,4(1h,3h)-pyrimidinedione
3. 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodo-unracil
4. 123i-fiau
5. 2'-fluoro-5-iodoarauracil
6. Fiau
1. 69123-98-4
2. Fiau
3. Fluoroiodoarauridine
4. 1-(2-deoxy-2-fluoro-b-d-arabinofuranosyl)-5-iodouracil
5. 53t7in77lc
6. 1-(2'fluoro-2'-deoxyarabinofuranosyl)-5-iodouracil
7. 1-[(2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione
8. Nsc-678514
9. 1-((2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-iodopyrimidine-2,4(1h,3h)-dione
10. Drg-0098
11. 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodouracil
12. Fialuridine [usan:inn]
13. Unii-53t7in77lc
14. Mfcd00866922
15. 2'-fluoro-5-iodo-1-beta-d-arabinofuranosyluracil
16. Uracil, 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodo-
17. Fialuridine [mi]
18. 2'-fluoro-5-iodouracil
19. Fialuridine [inn]
20. 5-iodo-2-fluoroarauracil
21. Fialuridine (usan/inn)
22. Fialuridine [usan]
23. Schembl3189
24. 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodo-2,4(1h,3h)-pyrimidinedione
25. 2,4(1h,3h)-pyrimidinedione, 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-iodo-
26. Fialuridine [mart.]
27. 1-(2-deoxy-2-fluoro-.beta.-d-arabinofuranosyl)-5-iodouracil
28. Chembl271475
29. Fialuridine, >=98% (hplc)
30. Dtxsid701028030
31. Zinc4216113
32. Bdbm50367488
33. Akos015856198
34. Akos016009483
35. Db15427
36. Nsc 678514
37. 1-[(2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-iodo-pyrimidine-2,4-dione
38. Ac-32361
39. As-69583
40. Hy-118122
41. Cs-0065233
42. D04181
43. F12931
44. 691f984
45. A848801
46. J-700174
47. J-700352
48. Q5446286
49. 1-(2-deoxy-2-fluoro--d-arabinofuranosyl)-5-iodouracil
50. 1-(2'-deoxy-2'-fluoro-.beta.-d-arabinofuranosyl)-5-iodouracil
51. 1-(2'-deoxy-2'-fluoro-b-d-arabinofuranosyl)-5-iodouracil
52. 2,4(1h,3h)-pyrimidinedione, 1-(2-deoxy-2-fluoro-.beta.-d-arabinofuranosyl)-5-iodo-
53. 1-((2s,3r,4s,5s)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-iodopyrimidine-2,4(1h,3h)-dione
54. 1-[(2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodo-1,2,3,4-tetrahydropyrimidine-2,4-dione
| Molecular Weight | 372.09 g/mol |
|---|---|
| Molecular Formula | C9H10FIN2O5 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 371.96185 g/mol |
| Monoisotopic Mass | 371.96185 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 418 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)