
1. Carbine
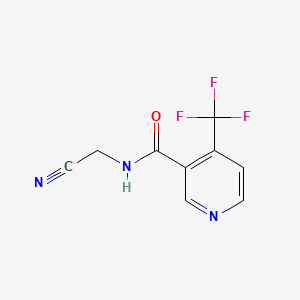
2. N-cyanomethyl-4-trifluoromethylnicotinamide
1. 158062-67-0
2. Aria
3. Flonicamid [iso]
4. N-(cyanomethyl)-4-(trifluoromethyl)nicotinamide
5. 3-pyridinecarboxamide, N-(cyanomethyl)-4-(trifluoromethyl)-
6. N-(cyanomethyl)-4-(trifluoromethyl)pyridine-3-carboxamide
7. N-(cyanomethyl)-4-(trifluoromethyl)-3-pyridinecarboxamide
8. Chebi:39291
9. 9500w2z53j
10. Iki 220
11. F 1785
12. Unii-9500w2z53j
13. Hsdb 7937
14. Beleaf
15. Turbine
16. Flonicamid [mi]
17. Schembl26954
18. Chembl1882892
19. Dtxsid8034611
20. 3-pyridinecarboxamide,n-(cyanomethyl)-4-(trifluoromethyl)-
21. Amy3555
22. Iki-220
23. Glxc-25868
24. Bcp29996
25. Zinc13827887
26. Akos015909866
27. Flonicamid 100 Microg/ml In Methanol
28. Ncgc00163878-01
29. As-75470
30. Flonicamid 100 Microg/ml In Acetonitrile
31. N-cyanomethyl-4-trifluoromethylnicotinamide
32. Db-043376
33. Flonicamid 1000 Microg/ml In Acetonitrile
34. Cs-0077202
35. F1309
36. Ft-0631185
37. N-cyanomethyl-4-(trifluoromethyl)nicotinamide
38. Flonicamid, Pestanal(r), Analytical Standard
39. C18463
40. D95873
41. F-1785
42. Q4328316
43. N-(cyanomethyl)-4-(trifluoromethyl)pyridine-3-carboxamide;3-pyridinecarboxamide, N-(cyanomethyl)-4-(trifluoromethyl)-
| Molecular Weight | 229.16 g/mol |
|---|---|
| Molecular Formula | C9H6F3N3O |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 229.04629631 g/mol |
| Monoisotopic Mass | 229.04629631 g/mol |
| Topological Polar Surface Area | 65.8 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 307 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
Five CRL:CD (SD)IGS BR rats/sex/level were dosed by gavage in 0.75% methylcellulose suspension with single administrations of either low or high doses of flonicamid. Intended dose levels were 2 and 50 mg/kg for both the pilot excretion study and for the pilot pharmacokinetics study. By error, the actual mean administered doses for the pilot excretion study were 0.85 and 21 mg/kg, which was unlikely to have affected results. The pilot excretion study assessed exhaled CO2 as well as urine, cage washings, and feces at intervals of 24 hr or less for 7 days. No measurable CO2 was detected in exhaled air. Urine plus cage wash samples accounted for 89-92% of administered label. About 5-6% of administered label was found in feces. Only 2-3% of label resided in carcasses at day 7. ... Tmax was estimated to be 0.3 to 0.6 hr.
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Flonicamid (158062-67-0) p.13 (April 28, 2005). Available from, as of May 24, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
(14)C Flonicamid (radiolabelled = 98.5% pure; unlabelled = 99.7%) was administered by oral gavage to CRL:CD (SD)IGS BR rats at 0 (0.75% methylcellulose/HPLC Grade H2O; 1/sex/dose at 6 and 168 hr termination), 2 mg/kg (3/sex/time point at 0.5, 6, 24 hours and 5/sex at 168 hour termination) and 400 mg/kg (3/sex/time point at 0.5, 6, 24 hours and 5/sex at 168 hour termination) to determine elimination and distribution. At 2 and 400 mg/kg, (14)C Flonicamid radioactivity was rapidly absorbed and excreted. A quantitative recovery was achieved during the 168 hour collection period. Urine contained 90% (including cage wash) of administered radioactivity, the majority of which was obtained within 24 hours of dosing at 2 mg/kg and by 48 hours at 400 mg/kg. Fecal elimination at 2 and 400 mg/kg was 5% of administered dose. In tissues, radioactivity levels increased rapidly with maximum concentrations mirroring those observed in the blood. While radioactivity was observed at all early time points in tissues, by 168 hours the levels had (where detectable) decreased by 50 - 100 fold. By 168 hours the carcasses contained 2% of radioactivity and liver had the highest tissue content (< 0.15%). At 2 mg/kg the greatest concentrations of radioactivity at 0.5 hours post dose for males and females respectively in liver (2.54-2.50 ug eq/g), kidney (2.35-2.67 ug eq/g), adrenals (5.07-6.52 ug eq/g), thyroid (4.02-4.26 ug eq/g) and ovaries (females - 3.77 ug eq/g). At 400 mg/kg males had the greatest concentration of radiolabel at 3 hours post dose in the liver (442 ug eq/g), kidney (311 ug eq/g), adrenals (672 ug eq/g) and thyroid (652 ug eq/g). Females had the greatest radiolabel concentrations at 1 hour post dose for liver (325 ug eq/g), kidney (359 ug eq/g), adrenals (689 ug eq/g) and thyroid (782 ug eq/g).
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Flonicamid (158062-67-0) p.14 (April 28, 2005). Available from, as of May 24, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
(14)C Flonicamid (radiolabelled = 98.5% pure; unlabelled = 99.7%) was administered by oral gavage to CRL:CD (SD)IGS BR rats at 0 (0.75% methylcellulose/HPLC Grade H2O; 1/sex/dose), 2 and 400 mg/kg (4/sex/dose), followed by a 48 hour termination time. At 2 and 400 mg/kg, (14)C Flonicamid radioactivity was rapidly absorbed and excreted. A quantitative recovery was achieved during the 48 hour collection period. Urine contained 85% (including cage wash) of administered radioactivity at 2 mg/kg and 80% at 400 mg/kg, the majority of which was excreted within 24 hours of dosing. Biliary excretion was low (4% at 2 mg/kg and 5% at 400 mg/kg) and the majority of radiolabel was excreted within the first 24 hours. Low levels of radioactivity were in feces (3.5-5.0%) and carcass (2.0-3.2%) at 2 mg/kg and in feces (3.8%) and carcass (1.5-2.1%). Therefore, biliary excretion was not a significant route of elimination of radioactivity. Increasing dose level had little effect on the disposition of radioactivity and there was no accumulation of radioactivity in the residual carcass. No sex-related differences were observed in any of the parameters measured.
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Flonicamid (158062-67-0) p.15 (April 28, 2005). Available from, as of May 24, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
The metabolic profile of flonicamid in rats was determined from the 0-48 hour interval rat urine after single dose administration of (14)C- pyridyl-flonicamid by oral gavage in male and female Sprague-Dawley rats at levels of 2 or 400 mg/kg body weight. Flonicamid was the major component in male and female rats with 52-72% of administered dose and the major metabolite is 4-trifluoromethylnicotinamide, with 18-25% of administered dose. Minor metabolites identified were: 4-trifluoromethylnicotinamide N-oxide (3% of administered dose), Flonicamid N-oxide (2% of administered dose), 4-trifluoromethylnicotinamide (1% of administered dose), 4-trifluoromethylnicotinamide conjugate (0.52% of administered dose), OH-4-trifluoromethylnicotinamide (0.44% of administered dose), TFNA (0.36% of administered dose), and 4-trifluoromethylnicotinamide N-Oxide conjugates (0.30% of administered dose). TFNG was not detected in the urine. Analysis of flonicamid rat metabolism for repeated dosing gave the following results: Flonicamid (46-54% of administered dose) and 4-trifluoromethylnicotinamide (21-27% administered dose) were the major components found in rat urine following multiple low doses of (14)C- pyridyl-flonicamid.
USEPA; Flonicamid: Flonicamid Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2007-0338-0004. p.17 (December 21, 2007). Available from, as of June 7, 2011: https://www.regulations.gov/#!home
In liver samples, the major components in male rat liver following 0.5 and 6 hours were flonicamid (51% and 27% total radioactive residues, respectively) and N-(4-trifluoromethylnicotinoyl)glycine (24% and 8% of total radioactive residues, respectively). 4-Trifluoromethylnicotinamide was 10% of total radioactive residues after 0.5 hours and 45% after 6 hours. In the rat biliary study, flonicamid was rapidly absorbed and excreted in the urine within 24 hours. ... The metabolic pathway of flonicamid in rats involves hydrolysis of the cyano (-CN) and amide (-CONH2) functional groups in the flonicamid molecule, although in rats, flonicamid was further metabolized by several routes, including N-oxidation and hydroxylation of the pyridine ring, leading to multiple metabolites.
USEPA; Flonicamid: Flonicamid Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2007-0338-0004. p.17 (December 21, 2007). Available from, as of June 7, 2011: https://www.regulations.gov/#!home
(14)C Flonicamid (radiolabelled = 98.5% pure; unlabelled = 99.7%) was used in 3 experiments in order to characterize metabolism in CRL:CD (SD)IGS BR rats: Study #1(Biliary): 4 rats/sex/dose were administered a single oral gavage dose of (14)C Flonicamid at 2 or 400 mg/kg, then terminated at 48 hours. Study #2 (Single-Dose Excretion): 3 or 5/sex/dose/time point were treated with a single oral gavage dose of (14)C Flonicamid at 2 or 400 mg/kg and terminated at 0.5, 6, 24 and 168 hours (2 mg/kg) or 3 (M), 1 (F), 14.5 (M), 8 (F), 24 and 168 hours. Study #3 (Multi-Dose Excretion): 2/sex/dose/time point were treated with 14 consecutive oral gavage doses of (12)C Flonicamid at 2 mg/kg, then one dose of (14)C Flonicamid on the 15th day before termination at 0.5, 6, 24 and 168 hours following (14)C Flonicamid administration. The negative control and vehicle was 0.75% methylcellulose/HPLC Grade H2O. Livers were collected and analyzed for metabolites in study #2 and #3. Excretion of Flonicamid and metabolites occurred primarily in the urine and to a lesser extent in the feces. It was metabolized by several routes, including nitrile hydrolysis, amide hydrolysis, N-oxidation and hydroxylation of the pyridine ring. Combinations of pathways occurred, leading to the formation of multiple metabolites.
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Flonicamid (158062-67-0) p.14 (April 28, 2005). Available from, as of May 24, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
The metabolism of flonicamid was investigated in livestock using lactating goats and laying hens. The test substance was [14C] flonicamid (labeled at the 3 position of the pyridine ring; specific activity 100,000 dpm/ug). In goats, the test substance was administered orally at 10 ppm (4.2x) in the diet for five consecutive days. Milk was collected twice daily throughout the study, and tissues (liver, kidney, muscle, and fat) were collected at sacrifice. In hens, the test substance was also administered orally at 10 ppm (25x) in the diet for five consecutive days. Eggs were collected twice daily throughout the study, and tissues (liver, muscle, skin, and fat) were collected at sacrifice. The available data indicate that the metabolism of flonicamid is similar in goats and hens. The majority of the dose was rapidly excreted. TFNA-AM (4-trifluoromethylnicotinamide) was the major metabolite (29-92% TRR) in goats (tissues and milk) and in laying hens (tissues and eggs). Flonicamid was found in minor quantities in goat and hen matrices, at <6% TRR. TFNAAM was also identified in goat muscle, liver, and kidney in significant quantities (23-31% TRR) in the acid hydrolysates of nonextractable residues. A metabolite determined to be an unstable conjugate of TFNA was identified in goat kidney at 12% TRR and the metabolite OH-TFNAAM was identified in liver acid hydrolysate at 11% /total residues recovered/ (TRR). The metabolism of flonicamid in livestock shows the main pathway of metabolism involves hydrolysis of the cyano and amide functional groups in the molecule ...
USEPA; Flonicamid: Flonicamid Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2007-0338-0004. p.17 (December 21, 2007). Available from, as of June 7, 2011: https://www.regulations.gov/#!home
(14)C Flonicamid (radiolabelled = 98.5% pure; unlabelled = 99.7%) was administered by gavage to CRL:CD (SD)IGS BR rats at 0 (0.75% methylcellulose suspension, 1/sex), 2 and 400 mg/kg (5/sex/dose). Blood samples were taken at 0, 10, 20 and 40 minutes and at 1, 2, 3, 4, 8, 24, 48 and 72 hours (terminated at 72 hours) to determine pharmacokinetics. After treatment, Flonicamid was rapidly absorbed and peak plasma radioconcentrations were rapidly achieved. Pharmacokinetics at 2 mg/kg were similar between the sexes but were different at 400 mg/kg. Females had a half-life of 6.8 hours after 400 mg/kg treatment. This was similar to the 4.5 hour half-life after treatment with 2 mg/kg. The average half-life in males at 2 mg/kg was 5.2 hours (similar to females), however at 400 mg/kg the plasma concentrations reached a plateau that lasted several hours (average half-life = 11.6 hours) in males and was statistically significantly different than high dose females and low dose males.
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Flonicamid (158062-67-0) p.13 (April 28, 2005). Available from, as of May 24, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm