


1. N-(2,6-difluorophenyl)-5-methoxy-8-fluoro-(1,2,4)-triazolo-(1,5c)-pyrimidine-2-sulfonamide
1. 145701-23-1
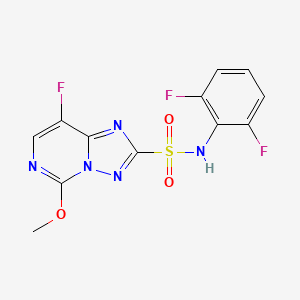
2. N-(2,6-difluorophenyl)-8-fluoro-5-methoxy-[1,2,4]triazolo[1,5-c]pyrimidine-2-sulfonamide
3. Primus
4. Kantor
5. Florasulam [iso]
6. Kantor; Primus; Primus Sc
7. 00a64zx8nb
8. Chebi:82009
9. Florasulam 10 Microg/ml In Acetonitrile
10. [1,2,4]triazolo[1,5-c]pyrimidine-2-sulfonamide, N-(2,6-difluorophenyl)-8-fluoro-5-methoxy-
11. N-(2,6-difluorophenyl)-8-fluoro-5-methoxy[1,2,4]triazolo[1,5-c]pyrimidine-2-sulfonamide
12. Unii-00a64zx8nb
13. Hsdb 7941
14. (1,2,4)triazolo(1,5-c)pyrimidine-2-sulfonamide, N-(2,6-difluorophenyl)-8-fluoro-5-methoxy-
15. N-(2,6-difluorophenyl)-8-fluoro-5-methoxy(1,2,4)triazolo(1,5-c)pyrimidine-2-sulfonamide
16. Nikos
17. Florasulam [mi]
18. Dsstox_cid_24340
19. Dsstox_rid_80157
20. Dsstox_gsid_44340
21. Schembl116614
22. Chembl3185236
23. Dtxsid7044340
24. Amy12231
25. Tox21_302433
26. Zinc38276325
27. Akos015901201
28. Db15316
29. Ds-6608
30. N-(2,6-difluorophenyl)-5-methoxy-8-fluoro-(1,2,4)-triazolo-(1,5c)-pyrimidine-2-sulfonamide
31. Ncgc00255762-01
32. Florasulam 100 Microg/ml In Acetonitrile
33. Db-042799
34. Florasulam 1000 Microg/ml In Acetonitrile
35. Cas-145701-23-1
36. Cs-0013914
37. Ft-0631173
38. Florasulam, Pestanal(r), Analytical Standard
39. C18850
40. 701f231
41. A884585
42. A1-01909
43. J-008140
44. Q22807578
45. 2',6',8-trifluoro-5-methoxy(1,2,4)triazolo(1,5-c)pyrimidine-2-sulfonanilide
46. [1,2,4]triazolo[1,5-c]pyrimidine-2- Sulfonamide, N-(2,6-difluorophenyl)-8-fluoro-5-methoxy-
| Molecular Weight | 359.29 g/mol |
|---|---|
| Molecular Formula | C12H8F3N5O3S |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 359.02999479 g/mol |
| Monoisotopic Mass | 359.02999479 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 541 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Rapidly absorbed (following oral administration), with about 91% excretion within 24 hr, mainly in the urine, and mainly as unchanged florasulam.
MacBean C, ed. Spirodiclofen (145701-23-1). In: The e-Pesticide Manual, 15th Edition, Version 5.0.1 (2010). Surrey UK, British Crop Protection Council.
In a dermal penetration study, (14)C-XDE-570 (Florasulam; 98-99% radiochemical purity as applied) was applied to the skin (12 sq cm) of Fischer 344 rats (4 males for each time point at each dose level). Nominal doses were 0.001 or 0.5 mg/sq cm skin. The high dose (EF-1343 commercial formulation) was included to assess exposure to mixer/loaders. The low dose (spray dilution, using an EF-1343 blank as a vehicle) represented a dose that was 2.39-fold more concentrated than the highest anticipated spray concentration for use on field crops, which was necessary in order to provide sufficient analytical sensitivity. The exposure duration was 24 hours, after which one group of 4 males for each dose level was sacrificed. The remaining 2 groups/dose were sacrificed at 48 or 72 hours post-application. Recovery of the applied dose (mass balance) was 100-103%. The majority of the dose was recovered in the skin swab (71-90% of the applied dose). Dermal absorption (based on the sum of residues in urine, feces, cage wash, tissues, residual carcass, and untreated skin) was only 0.13-0.45% of the applied dose and only 10-22% of the applied dose remained in the skin at the application site (considered potentially absorbable). Increasing the dose 200-fold resulted in only approximately 2-fold increase in absorption. Absorption increased 44% at 48 hr and 61% at 72 hr compared to 24 hr in the low dose groups; however, a time-dependent increase in absorption was not evident in the high dose groups. The absorbed dose was almost completely excreted in the urine at the low dose, but was found primarily in the urine, cage wash, and untreated skin at the high dose. The amount of radioactivity at the treatment site increased at 48 hours in the low dose, but did not decrease within 72 hours at either dose, suggesting that the compound in the skin was not readily absorbable. The compound isolated in the treated skin after 72 hours (including the 24 hour exposure period) would be absorbed in negligible amounts. The highest dermal absorption noted was 0.45% of the applied dose. This value is considered appropriate for use in risk assessment, with the appropriate uncertainty factors applied.
USEPA; Florasulam: Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2006-0993-0004. p.53-4 (May 31, 2007). Available from, as of May 25, 2011: https://www.regulations.gov/#!home
(14)C-XDE-570 (Florasulam; 99.3-99.5% radiochemical purity) in a suspension of 0.5% Methocel cellulose ethers was administered to 5 Fischer 344 rats/sex as a single gavage dose at 10 or 500 mg/kg bw. Additionally, 5 rats/sex were treated with 14 daily doses at 10 mg/kg bw/day of non-labeled florasulam followed by a single oral dose of (14)C-florasulam on Day 15. (14)C-Florasulam was uniformly labeled in the aniline ring for each of these test groups. In addition, 5 males were treated with a single gavage dose at 10 mg/kg bw with (14)C-florasulam (labeled at the 9 position in the triazolo-pyrimidine ring). All animals were killed 168 hours after the administration of the radiolabeled dose. Absorption was rapid and extensive. Approximately 90-93% of the dose was absorbed in the 10 mg/kg rats, and 82-86% was absorbed in the 500 mg/kg rats (based on the sum of radioactivity detected in the urine, tissues/carcass, and cage rinse). Peak plasma concentrations (Cmax) were achieved within 0.5-1 hour following dose administration at 10 or 500 mg/kg. Cmax in the plasma did not increase proportionally with dose, possibly indicating a saturation of the absorption and/or excretion mechanisms at the high dose. The apparent volume of distribution was increased at the high dose, possibly indicative of increased tissue binding. Total recoveries at 168 hours post-dose were 95.9-100.2% of the administered dose. Elimination was rapid. The administered dose was mostly eliminated within 12 hours in the urine (>80% of the dose at 10 mg/kg and >60% of the dose at 500 mg/kg). Total radioactivity found in the urine was approximately 90-92% of the dose following single or repeated low-dose treatment, and 81-85% of the dose following treatment at 500 mg/kg. Radioactivity in the feces accounted for another 5-7% at 10 mg/kg and 14-17% at 500 mg/kg. Thus, compared to the low dose, excretion of the high dose was slightly slower, and more of the compound was excreted in the feces. At 24 hours, <0.5% of the dose was found in expired air. By 24 hours post-dose, plasma levels had declined to <0.1ug eq/g plasma in both sexes at 10 mg/kg and <5.0 ug eq/g plasma in both sexes at 500 mg/kg. The highest residue levels were observed in the skin (single dose) and carcass (repeated dose), but the mean recovery of radioactivity in the tissues/carcass at sacrifice was <0.6% of the dose.
USEPA; Florasulam: Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2006-0993-0004. p.52-3 (May 31, 2007). Available from, as of May 25, 2011: https://www.regulations.gov/#!home
(14)C-XDE-570 (Florasulam; 99.3-99.5% radiochemical purity) in a suspension of 0.5% Methocel cellulose ethers was administered to 5 Fischer 344 rats/sex as a single gavage dose at 10 or 500 mg/kg bw. Additionally, 5 rats/sex were treated with 14 daily doses at 10 mg/kg bw/day of non-labeled florasulam followed by a single oral dose of (14)C-florasulam on Day 15. (14)C-Florasulam was uniformly labeled in the aniline ring for each of these test groups. In addition, 5 males were treated with a single gavage dose at 10 mg/kg bw with (14)C-florasulam (labeled at the 9 position in the triazolo-pyrimidine ring). All animals were killed 168 hours after the administration of the radiolabeled dose. ... Identified compounds accounted for 87.6-91.6% of the administered dose in each group. In each group, the following compounds were isolated: parent accounted for 77.7-85.0% dose, OH-phenyl- XR-570 (exact position of hydroxyl group not determined) accounted for 3.1-9.0% dose, OH-phenyl-XR-570 sulfate conjugate accounted for 2.8-3.7% dose, and 2 unidentified metabolites accounted for < or = 0.32% dose. In the high dose, more of the parent was isolated in the feces and less in the urine compared to the low dose.
USEPA; Florasulam: Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2006-0993-0004. p.52-3 (May 31, 2007). Available from, as of May 25, 2011: https://www.regulations.gov/#!home
Florasulam is a selective triazolopyrimidine sulfonanilide post-emergent herbicide. The pesticidal mode of action (MOA) is through inhibition of acetolactate synthase (ALS) in plants. ALS is found in the chloroplast where it catalyses branch chained amino acid biosynthesis. Inhibition of ALS results in inhibition of plant cell division, decreased plant growth, and ultimately, plant death.
USEPA; Florasulam: Human Health Risk Assessment. Document ID: EPA-HQ-OPP-2006-0993-0004. p.11 (May 31, 2007). Available from, as of May 25, 2011: https://www.regulations.gov/#!home