
1. 5 Fluorodeoxyuridine
2. 5-fluorodeoxyuridine
3. 5-fudr
4. Fluorodeoxyuridine
5. Fudr
1. 50-91-9
2. 5-fluorodeoxyuridine
3. 2'-deoxy-5-fluorouridine
4. Fluorodeoxyuridine
5. Fudr
6. 5-fluoro-2'-deoxyuridine
7. Fdurd
8. Deoxyfluorouridine
9. Fluoruridine Deoxyribose
10. Floxuridin
11. 5fdu
12. Floxuridinum
13. Floxiridina
14. 5-fluoro-2-desoxyuridine
15. 5-fluorouracil 2'-deoxyriboside
16. 5 Fluorodeoxyuridine
17. Beta-5-fluoro-2'-deoxyuridine
18. Floxuridinum [inn-latin]
19. Floxiridina [inn-spanish]
20. 5-fluoro-1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1h,3h)-dione
21. 1-(2-deoxy-beta-d-ribofuranosyl)-5-fluorouracil
22. 5-fluoro-2-deoxyuridine
23. Uridine, 2'-deoxy-5-fluoro-
24. 5-fluorouracil Deoxyriboside
25. Nsc-27640
26. 1beta-d-2'-deoxyribofuranosyl-5-flurouracil
27. 1-beta-d-2'-deoxyribofuranosyl-5-flurouracil
28. 5-fudr
29. Fdur
30. (+)-5-fluoro-2'-deoxyuridine
31. Chebi:60761
32. (+)-5-fluorodeoxyuridine
33. 5-fdurd
34. 5-fluoro-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,3,4-tetrahydropyrimidine-2,4-dione
35. Chembl917
36. Mls000069439
37. 039lu44i5m
38. Nsc 27640
39. Uridine, 2'-deoxy-5'-fluoro-
40. Ncgc00023722-05
41. Smr000059051
42. Dsstox_cid_3057
43. Ro 5-0360
44. Dsstox_rid_76855
45. Dsstox_gsid_23057
46. Fdu
47. Cas-50-91-9
48. 5-fluoro-1-((2r,4s,5r)-4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1h-pyrimidine-2,4-dione
49. Hsdb 3227
50. Einecs 200-072-5
51. Mfcd00006530
52. Fudr (tn)
53. 5-fluoro-2'-deoxy-beta-uridine
54. Brn 0090221
55. Floxidine
56. Uridine, 2'-deoxy-5-fluoro
57. Nsc27640
58. Unii-039lu44i5m
59. Floxuridine [usan:usp:inn]
60. Ai3-50691
61. 5-floxuridine
62. Floxuridine,(s)
63. 5-fluoro-durd
64. Floxuridine (fludara)
65. Floxuridine (usp/inn)
66. Floxuridine [mi]
67. Opera_id_1559
68. Floxuridine [inn]
69. 2-deoxy-5'-fluorouridine
70. Floxuridine [hsdb]
71. Floxuridine [usan]
72. Epitope Id:138108
73. 5-fluor-1-(beta-2'-deoxyribofuranosyl)pyrimidin-2,4(1h,3h)-dion [czech]
74. Floxuridine [vandf]
75. Schembl4424
76. 2''-deoxy-5-fluorouridine
77. 2'-deoxy-5-fluoro Uridine
78. 5-fluoro-2''-deoxyuridine
79. Floxuridine [mart.]
80. Floxuridine [usp-rs]
81. Floxuridine [who-dd]
82. 5-24-06-00291 (beilstein Handbook Reference)
83. Mls002695934
84. Mls006011768
85. 5-fluoro-2''-deoxy-uridine
86. 2'-deoxy-5-fluoro-d-uridine
87. Gtpl4801
88. Dtxsid3023057
89. Floxuridine [orange Book]
90. Floxuridine [usp Impurity]
91. Hms2090i12
92. Hms2230n22
93. Hms3715a11
94. Floxuridine [usp Monograph]
95. Bcp02871
96. Hy-b0097
97. Zinc3813010
98. Tox21_110892
99. 5-fluor-1-(beta-2'-deoxyribofuranosyl)pyrimidin-2,4(1h,3h)-dion
100. Bbl033765
101. Bdbm50340678
102. S1299
103. Stk801909
104. Akos005622662
105. Tox21_110892_1
106. Bcp9000691
107. Ccg-220965
108. Cs-1827
109. Db00322
110. Ps-7520
111. Sri-9246-15
112. Sri-9246_18
113. Ncgc00023722-03
114. Ncgc00023722-06
115. Ncgc00023722-08
116. 1-[(4s,2r,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-fluoro-1,3-dihydropyri Midine-2,4-dione
117. 136353-77-0
118. Ac-16391
119. Bcp0726000269
120. D2235
121. En300-52649
122. D04197
123. 006f530
124. Sr-01000000001
125. J-700034
126. Q5462356
127. Sr-01000000001-2
128. Brd-k47832606-001-26-9
129. Brd-k47832606-001-30-1
130. 5-fluoro-2'-deoxyuridine, Thymidylate Synthase Inhibitor
131. Z802671482
132. 1-(beta-d-2-deoxy-erythro-pentofuranosyl)-5-fluorouracil
133. Floxuridine, United States Pharmacopeia (usp) Reference Standard
134. 5-fluoro-1-(4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1h-pyrimidine-2,4-dione
135. 5-fluoro-1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)pyrimidine-2,4(1h,3h)-dione
136. 5-fluoro-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4-dione
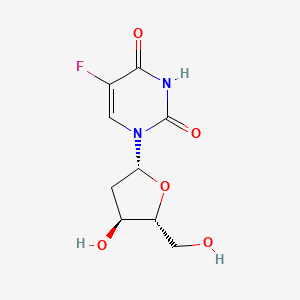
| Molecular Weight | 246.19 g/mol |
|---|---|
| Molecular Formula | C9H11FN2O5 |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 246.06519962 g/mol |
| Monoisotopic Mass | 246.06519962 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 386 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Floxuridine |
| PubMed Health | Floxuridine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Floxuridine for Injection, USP, an antineoplastic antimetabolite, is available as a sterile, nonpyrogenic, lyophilized powder for reconstitution. Each vial contains 500 mg of floxuridine which is to be reconstituted with 5 mL of sterile water for i... |
| Active Ingredient | Floxuridine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Floxuridine |
| PubMed Health | Floxuridine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Floxuridine for Injection, USP, an antineoplastic antimetabolite, is available as a sterile, nonpyrogenic, lyophilized powder for reconstitution. Each vial contains 500 mg of floxuridine which is to be reconstituted with 5 mL of sterile water for i... |
| Active Ingredient | Floxuridine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
Antimetabolites, Antineoplastic; Antiviral Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Floxuridine, given by continuous regional intra-arterial infusion, is indicated for palliative management of colorectal carcinoma metastatic to the liver that has not responded to other treatment. Floxuridine is most useful when the disease has not extended beyond an area capable of infusion via a single artery. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1477
Floxuridine also is indicated for carcinoma of the ovary and kidney not responsive to other antimetabolites. /Not Include in the US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1477
Floxuridine has also been used for carcinoma of the breast, ovary, cervix, urinary bladder, kidney, and prostate not responsive to other antimetabolites. /NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1334
Floxuridine is metabolized to fluorouracil, but the full spectrum of fluorouracil toxicity is not expected with floxuridine because of regional administration of the drug by intra-arterial infusion. However, the possibility of typical adverse effects of fluorouracil during floxuridine therapy should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1045
Nausea, vomiting, and diarrhea are common adverse effects; anorexia, cramps, and pain also may occur. Stomatitis is one of the most common signs of specific toxicity. Enteritis occurs frequently and duodenal ulcer, duodenitis, gastritis, gastroenteritis, glossitis, GI bleeding, and pharyngitis also have been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1045
Leukopenia and anemia occur commonly with floxuridine therapy; thrombocytopenia also may occur. The patient's hematologic status must be carefully monitored.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1045
Pancytopenia and agranulocytosis have been reported in patients receiving fluorouracil; because of its pharmacologic similarity, these adverse hematologic effects might occur in patients receiving floxuridine.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1045
For more Drug Warnings (Complete) data for FLOXURIDINE (25 total), please visit the HSDB record page.
For palliative management of gastrointestinal adenocarcinoma metastatic to the liver, when given by continuous regional intra-arterial infusion in carefully selected patients who are considered incurable by surgery or other means. Also for the palliative management of liver cancer (usually administered by hepatic intra-arterial infusion).
Floxuridine is an anti-metabolite or a pyrimidine analog that works by disrupting the process S-phase of cell division, selectively targeting rapidly dividing cells. Due to the structural similarities, antimetabolites act as pyrimidine-like molecules and prevent normal pyrimidines from being incorporated into DNA. After successful biotransformation, floxuridine is converted into an active component, flurouracil, which blocks the enzyme which converts cytosine nucleosides into the deoxy derivative. Flurouracil also physically prevents the incorporation of thymidine nucleotides into the DNA strand by taking their place, further preventing DNA synthesis.
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BC - Pyrimidine analogues
L01BC09 - Floxuridine
Route of Elimination
Floxuridine can be excreted as unchanged drug, urea, fluorouracil, a-fluoro-bureidopropionic acid, dihydrofluorouracil, a-fluoro-b-guanidopropionic acid and a-fluoro-b-alanine via the kidneys. Floxuridine may also be excreted as respiratory carbon dioxide.
... Floxuridine /is/ ... administered parenterally, since absorption after ingestion ... is unpredictable and incomplete.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1229
It is not known whether floxuridine is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1046
Some /Floxuridine/ crosses the blood-brain barrier; active metabolites are localized intracellularly.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1478
Elimination /is/ respiratory (as carbon dioxide), about 60%. Renal /elimination accounts for/ 10 to 13% (as unchanged drug and metabolites).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1478
Hepatic.
Biotransformation /is/ hepatic and in tissues, extensive, to the monophosphate derivative and fluorouracil; after continuous intra-arterial infusion, conversion to the monophosphate derivative is enhanced; largely converted to fluorouracil after rapid intravenous or intra-arterial injection.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1478
Following infusion of small doses of floxuridine, most of the drug appears to be anabolized to FUDR-MP, the active metabolite of the drug. When single doses are administered rapidly, floxuridine is apparently rapidly catabolized to fluorouracil.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1046
Floxuridine and fluorouracil are metabolized in the liver. Metabolic degradation of floxuridine is less when the drug is given by continuous infusion than when given by single injections. The drug is excreted intact and as urea, fluorouracil, a-fluoro--ureidopropionic acid, dihydrofluorouracil, alpha-fluoro-beta-guanidopropionic acid, and alpha-fluoro-beta-alanine in the urine and as respiratory carbon dioxide.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1046
Metabolic degradation occurs, particularly in the liver. Floxuridine is converted by thymidine or deoxyuridine phosphorylases into 5-fluorouracil. 5-Fluorouracil is inactivated by reduction of the pyrimidine ring; this reaction is carried out by dihydrouracil dehydrogenase, which is found in liver, intestinal mucosa, and other tissues. Inherited deficiency of this enzyme leads to greatly increased sensitivity to the drug. The product of this reaction, 5-fluoro-5,6-dihydrouracil is ultimately degraded to alpha-fluoro-beta-alanine ... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1229
Floxuridine rapidly undergoes catabolism to form 5-fluorouracil, which is the active component of the drug. 5-Fluorouracil primarily works by interfering with DNA synthesis; however, it may also inhibit the formation of fraudulent RNA via physical incorporation into the RNA. It is also an inhibitor of riboside phophorylase, preventing the utilization of pre-formed uracil in RNA synthesis. Floxuridine can also form 5-fluoro-2'-deoxyuridine-5'-phosphate (FUDR-MP), which is the monophosphate of floxuridine that inhibits thymidylate synthetase that plays a role in the methylation of deoxyuridylic acid to thymidylic acid during DNA synthesis. FUDR-MP thus interferes with DNA synthesis.
Floxuridine is an antimetabolite. The monophosphate of the drug, 5-fluoro-2'-deoxyuridine-5'-phosphate (FUDR-MP), inhibits thymidylate synthetase, thus inhibiting methylation of deoxyuridylic acid to thymidylic acid and thereby interfering with the synthesis of DNA. Following administration of a single dose of floxuridine, the drug is catabolized to fluorouracil and produces the same antimetabolic effects as the latter drug. In addition to interfering with DNA synthesis, metabolites of fluorouracil become incorporated to a small extent into RNA, producing a fraudulent RNA. Fluorouracil also inhibits utilization of preformed uracil in RNA synthesis by blocking uracil riboside phosphorylase.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 1046
Floxuridine is an antimetabolite of the pyrimidine analog type. Floxuridine is considered to be cell cycle-specific for the S-phase of cell division. Activity occurs as the result of activation in the tissue, and includes inhibition of DNA and, as a result of action of the fluorouracil metabolite, RNA synthesis.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1477
Fluorouracil requires enzymatic conversion to the nucleotide in order to exert its cytotoxic activity. Several routes are available for the formation of the 5'-monophosphate nucleotide in animal cells. Fluorouracil may be converted to fluorouridine by uridine phosphorylase and then to 5'-monophosphate nucleotide by uridine kinase or it may react directly with 5-phosphoribosyl- 1 -pyrophosphate, catalyzed by the enzyme orotate phosphoribosyl transferase, to form 5'-monophosphate nucleotide. Fluorouracil may also be converted directly to floxuridine by the enzyme thymidine phosphorylase. Many metabolic pathways are available to 5'-monophosphate nucleotide, including incorporation into RNA. A reaction sequence crucial for antineoplastic activity involves reduction of the diphosphate nucleotide by the enzyme ribonucleotide diphosphate reductase to the deoxynucleotide level and the eventual formation of 5-fluoro-2'-deoxyuridine-5'-phosphate. This complex metabolic pathway for the generation of the actual growth inhibitor, 5-fluoro-2'-deoxyuridine-5'-phosphate, may be bypassed through use of the deoxyribonucleoside of fluorouracil--floxuridine--which is converted directly to 5-fluoro-2'-deoxyuridine-5'-phosphate by thymidine kinase. However, floxuridine is a good substrate for both thymidine and uridine phosphorylases. and it can also be degraded to fluorouracil.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1228