

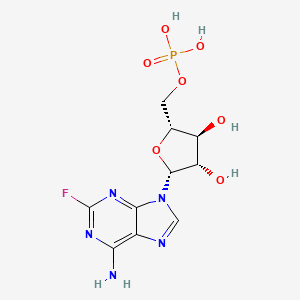

1. 9 Beta-d-arabinofuranosyl-2-fluoroadenine Monophosphate
2. 9h-purin-6-amine, 2-fluoro-9-(5-o-phosphono-beta-d-arabinofuranosyl)-
3. Beneflur
4. F-ara-amp
5. Faraamp
6. Fludara
7. Fludarabine 5'-monophosphate
8. Fludarabine Monophosphate
9. Fluoro-ara-amp
10. Nsc 312887
11. Nsc-312887
1. 75607-67-9
2. Fludara
3. Fludarabine 5'-monophosphate
4. Oforta
5. Fludarabine Monophosphate
6. 2-fluoro-ara Amp
7. 2-f-ara-amp
8. Fludarabine (phosphate)
9. Famp
10. Nsc-312887
11. 2-fluoro-9-(5-o-phosphono-beta-d-arabinofuranosyl)-9h-purin-6-amine
12. ((2r,3s,4s,5r)-5-(6-amino-2-fluoro-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl Dihydrogen Phosphate
13. Chebi:63599
14. 2-fluoroadenine Arabinoside 5'-monophosphate
15. 1x9vk9o1sc
16. 9-beta-arabinofuranosyl-2-fluoroadenine-5'-phosphate
17. 9-beta-d-arabinofuranosyl-2-fluoroadenine 5'-monophosphate
18. 75607-67-9 (phosphate)
19. 9-beta-d-arabinofuranosyl-2-fluoroadenine 5'-(dihydrogen Phosphate)
20. Fludarabine Phosphate (fludara)
21. {[(2r,3s,4s,5r)-5-(6-amino-2-fluoro-9h-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic Acid
22. Nsc 312887
23. Nsc 328002
24. [(2r,3s,4s,5r)-5-(6-amino-2-fluoropurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl Dihydrogen Phosphate
25. Nsc-328002
26. Smr002544683
27. F-ara-amp
28. Sr-05000001945
29. Unii-1x9vk9o1sc
30. Beneflur
31. Fludara Phosphate
32. Fludara (tn)
33. Fludarabine-phosphate
34. 2-fluoro-ara-amp
35. Mfcd00866418
36. Fludarabine Phosphate [usan:usp:ban]
37. Fludarabine Phosphate;
38. 2f-ara-amp
39. Schembl3511
40. Nsc 312887 Phosphate
41. Mls003915617
42. Mls004774150
43. 9-bata-d-arabinofuranosyl-2-fluoroadenine Phosphate
44. Fludarabine Phosphate(fludara)
45. 9-.beta.-d-arabinofuranosyl-2-fluoroadenine 5'-(dihydrogen Phosphate)
46. Chembl1096882
47. Dtxsid2023060
48. Fludarabine Phosphate (jan/usp)
49. Sht-586
50. Fludarabine For System Suitability
51. Hms2094o11
52. Pharmakon1600-01505705
53. Fludarabine Phosphate [jan]
54. Ex-a2028
55. Hy-b0028
56. Nsc-118218h
57. Zinc3927870
58. Fludarabine Phosphate [usan]
59. Bdbm50248004
60. Fludarabine Phosphate [vandf]
61. Hg1010
62. Nsc759194
63. S1229
64. Fludarabine Phosphate [mart.]
65. 9h-purin-6-amine, 2-fluoro-9-(5-o-phosphono-beta-d-arabinofuranosyl)-
66. Akos024464516
67. Fludarabine Phosphate [usp-rs]
68. Fludarabine Phosphate [who-dd]
69. Bcp9000694
70. Ccg-213521
71. Cs-0861
72. F-ara-a (nsc 312887) Phosphate
73. Nsc-759194
74. Sri-5907-04
75. Sri-5907_05
76. Sri-5907_07
77. As-14202
78. Bcp0726000268
79. Fludarabine 5'-monophosphate [mi]
80. Fludarabine Phosphate [orange Book]
81. Sbi-0206893.p001
82. Fludarabine Phosphate [ep Monograph]
83. Fludarabine Phosphate [usp Monograph]
84. Sw218146-2
85. D01907
86. 607f679
87. A838460
88. Q185916
89. Sr-05000001945-1
90. Sr-05000001945-4
91. Z2235802254
92. 9-beta-d-arabinofuranosyl-2-fluoroadenine-5'-monophosphate
93. 9h-purin-6-amine, 2-fluoro-9-(5-o-phosphono-b-d-arabinofuranosyl)-
| Molecular Weight | 365.21 g/mol |
|---|---|
| Molecular Formula | C10H13FN5O7P |
| XLogP3 | -3.1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 4 |
| Exact Mass | 365.05366293 g/mol |
| Monoisotopic Mass | 365.05366293 g/mol |
| Topological Polar Surface Area | 186 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 514 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Fludarabine phosphate |
| Drug Label | Fludarabine Phosphate Injection contains fludarabine phosphate, a fluorinated nucleotide analog of the antiviral agent vidarabine, 9- D-arabinofuranosyladenine (ara-A) that is relatively resistant to deamination by adenosine deaminase. Each mL cont... |
| Active Ingredient | Fludarabine phosphate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/2ml (25mg/ml); 50mg/vial |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa; Sandoz; Actavis Elizabeth; Mustafa Nevzat Ilac; Fresenius Kabi Usa; Onco Therapies |
| 2 of 2 | |
|---|---|
| Drug Name | Fludarabine phosphate |
| Drug Label | Fludarabine Phosphate Injection contains fludarabine phosphate, a fluorinated nucleotide analog of the antiviral agent vidarabine, 9- D-arabinofuranosyladenine (ara-A) that is relatively resistant to deamination by adenosine deaminase. Each mL cont... |
| Active Ingredient | Fludarabine phosphate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/2ml (25mg/ml); 50mg/vial |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa; Sandoz; Actavis Elizabeth; Mustafa Nevzat Ilac; Fresenius Kabi Usa; Onco Therapies |
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
