
1. Apurone
2. R 802
3. R-802
1. 42835-25-6
2. Apurone
3. Flumigal
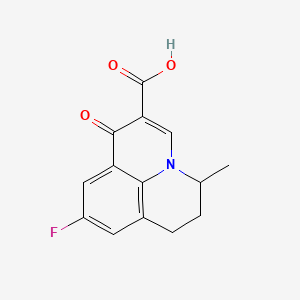
4. 9-fluoro-5-methyl-1-oxo-1,5,6,7-tetrahydropyrido[3,2,1-ij]quinoline-2-carboxylic Acid
5. R-802
6. Uvg8vsp2sj
7. Nsc-757806
8. Mls000069546
9. Chebi:85269
10. 1h,5h-benzo(ij)quinolizine-2-carboxylic Acid, 9-fluoro-6,7-dihydro-5-methyl-1-oxo-
11. 9-fluoro-5-methyl-1-oxo-6,7-dihydro-1h,5h-pyrido[3,2,1-ij]quinoline-2-carboxylic Acid
12. Ncgc00018211-03
13. Flumequinum
14. Flumequino
15. Flumiquil
16. Flumisol
17. Imequyl
18. Smr000058826
19. Flumix
20. R 802 (bactericide)
21. 1h,5h-benzo[ij]quinolizine-2-carboxylic Acid, 9-fluoro-6,7-dihydro-5-methyl-1-oxo-
22. Dsstox_cid_25623
23. Dsstox_rid_81008
24. Dsstox_gsid_45623
25. 7-fluoro-12-methyl-4-oxo-1-azatricyclo[7.3.1.05,13]trideca-2,5,7,9(13)-tetraene-3-carboxylic Acid
26. Flumequinum [inn-latin]
27. Flumequino [inn-spanish]
28. Flm
29. Hsdb 7034
30. R 802
31. Sr-01000000130
32. Unii-uvg8vsp2sj
33. Einecs 255-962-6
34. Fantacin
35. Flumequine [usan:inn:ban]
36. (rs)-9-fluoro-5-methyl-1-oxo-6,7-dihydro-1h,5h-benzo[i,j]quinolizine-2-carboxylic Acid
37. Prestwick_603
38. Apurone (tn)
39. Cas-42835-25-6
40. Mfcd00079298
41. Spectrum_000367
42. Flumequine [mi]
43. Flumequine [inn]
44. Flumequine (usan/inn)
45. Opera_id_1399
46. Prestwick0_000204
47. Prestwick1_000204
48. Prestwick2_000204
49. Prestwick3_000204
50. Spectrum2_001200
51. Spectrum3_001417
52. Spectrum4_000826
53. Spectrum5_001532
54. Flumequine [hsdb]
55. Flumequine [usan]
56. Flumequine [mart.]
57. 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1h,5h-benzo(ij)quinolizine-2-carboxylic Acid
58. Flumequine [who-dd]
59. Schembl49829
60. Bspbio_000187
61. Bspbio_003094
62. Kbiogr_001371
63. Kbioss_000847
64. Mls001074120
65. Divk1c_000089
66. Spectrum1500992
67. Spbio_001279
68. Spbio_002108
69. Bpbio1_000207
70. Chembl370252
71. Dtxsid5045623
72. Chebi:94431
73. Hms500e11
74. Kbio1_000089
75. Kbio2_000847
76. Kbio2_003415
77. Kbio2_005983
78. Kbio3_002314
79. Flumequine [ep Monograph]
80. Ninds_000089
81. Hms1568j09
82. Hms1921o10
83. Hms2092o08
84. Hms2095j09
85. Hms2230f14
86. Hms3373o15
87. Hms3652k09
88. Hms3712j09
89. Pharmakon1600-01500992
90. Hy-b0526
91. Tox21_110840
92. Ccg-40315
93. Nsc757806
94. S3181
95. Fluoro-methyl-oxo-[?]carboxylic Acid
96. 6,7-dihydro-9-fluoro-5-methyl-1-oxo-1h,5h-quinolizine-2-carboxylic Acid
97. Akos015904879
98. Tox21_110840_1
99. Ac-1284
100. Db08972
101. Hs-0096
102. Nsc 757806
103. Idi1_000089
104. Flumequin 100 Microg/ml In Acetonitrile
105. Ncgc00018211-02
106. Ncgc00018211-04
107. Ncgc00018211-05
108. Ncgc00018211-06
109. Ncgc00018211-10
110. Ncgc00089803-02
111. Ncgc00089803-03
112. Ncgc00178300-01
113. Ncgc00178300-02
114. Sbi-0051623.p002
115. Flumequine 1000 Microg/ml In Acetonitrile
116. F0832
117. Ft-0626439
118. Sw196774-3
119. C75146
120. D02302
121. Flumequine, Vetranal(tm), Analytical Standard
122. Ab00052187_17
123. Ab00052187_18
124. 835f256
125. A872809
126. Q-201123
127. Q3074500
128. Sr-01000000130-2
129. Sr-01000000130-4
130. Brd-a69777949-001-05-1
131. Brd-a69777949-001-15-0
132. Flumequine, European Pharmacopoeia (ep) Reference Standard
133. 1h,5h-benzo[ij]quinolizine-2-carboxylic Acid, 9-fluoro-6,7-dihydro-5-methyl-1-oxo
134. 6,7-dihydro-9-fluoro-5-methyl-1-oxo-1h,5h-benzo(i,j)quinolizine-2-carboxylic Acid
135. 6,7dihydro-9-fluoro-5-methyl-1-oxo-1h,5h-benzo[ij]-quinolizine-2-carboxylic Acid
136. 9-fluoro-1,5,6,7-tetrahydro-5-methyl-1-oxopyrido[3,2,1-ij]quinoline-2-carboxylic Acid
137. 7-fluoro-12-methyl-4-oxo-1-azatricyclo[7.3.1.0^{5,13}]trideca-2,5,7,9(13)-tetraene-3-carboxylic Acid
138. Flumequine Solution; 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1h,5h-benzo[ij]quinolizine-2-carboxylic Acid
| Molecular Weight | 261.25 g/mol |
|---|---|
| Molecular Formula | C14H12FNO3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 261.08012141 g/mol |
| Monoisotopic Mass | 261.08012141 g/mol |
| Topological Polar Surface Area | 57.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Urinary; Topoisomerase II Inhibitors
National Library of Medicine's Medical Subject Headings. Flumequine. Online file (MeSH, 2015). Available from, as of August 20, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Flumequine is a fluoroquinolone compound with antimicrobial activity against Gram-negative organisms. It is used in the treatment of enteric infections in food animals and in the treatment of bacterial infections in farmed fish. Flumequine also has limited use in humans for the treatment of urinary tract infections.
WHO/FAO; WHO Technical Report Series 939, Evaluation of Certain Veterinary Drug Residues in Food, 66th Report of the Joint FAO/WHO Expert Committee on Food Additives (2006). Available from, as of October 13, 2015: https://apps.who.int/iris/bitstream/10665/43464/1/9241209399_eng.pdf
The efficacy and safety of flumequine were evaluated in the treatment of 121 cases uncomplicated (65.5%) and complicated (34.5%) urinary tract infections (UTI) when given as a dose of 400 mg bd. Duration of treatment ranged from 7-15 days, with a mean of 10. Thirty days post-therapy, cure persisted in 92.3% of the patients with uncomplicated UTI and in 53.7% of those with complicated UTI. Relapse or re-infection occurred in 34.1% of the patients with complicated UTI, and in 12.2%, the infecting organism did not respond to treatment. Flumequine was generally well tolerated. In 27.3% of patients gastrointestinal, and neurological disorders and skin rashes developed which in most cases were mild. Only two patients were withdrawn from the treatment. It is concluded that flumequine, administered at 800 mg daily, is highly effective in treating uncomplicated and complicated UTI.
PMID:3356617 Schena FP et al; J Antimicrob Chemother 21 (1): 101-6 (1988)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MB - Other quinolones
J01MB07 - Flumequine
Peak plasma levels occurred in male dogs between 2 and 4 hours after dosing. Peak plasma levels were approximately 55-65 ug flumequine equivalents/mL of plasma after an oral dose of 25 mg/kg bw. Approximately one-half the concentration of total radioactivity for the first 12 hours following administration corresponded to unchanged drug. The disappearance of flumequine from the plasma appeared to follow multi-exponential kinetics with an initial half-life of about 75 minutes and a terminal beta-phase half-life of 6.5 hours.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
Studies with (14)C-flumequine in dogs and rats indicated that flumequine is readily absorbed following oral administration.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
There was a significant difference in the mode of drug excretion between dogs and rats. In dogs, 55-75% of the dose was excreted in the faeces compared to only 10-15% in rats. Less than 5% of the dose was present in the urine of dogs as unchanged drug while another 13-15% was excreted as a conjugate of flumequine. In rats, 20-36% of the dose was excreted in urine as unchanged drug and very little as a conjugate of flumequine. The concentrations of free flumequine in the 24-hour urine sample were about the same for both species.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
Total recovery of the orally administered dose was achieved in the urine and feces within 5 days after dosing in both species /rats and dogs/, indicating that very little residual flumequine and/or metabolites were retained in the tissues.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for FLUMEQUINE (8 total), please visit the HSDB record page.
In dogs, less than 5% of the dose was excreted in the urine as unchanged drug and 13-15% was excreted as an acid-labile urinary conjugate of flumequine (or a material fluorometrically similar to flumequine). In rats, 20-36% was excreted in the urine as unchanged drug and very little as an acid-labile conjugate.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
In a 13-week study designed to investigate hepatotoxic lesions and the activities of hepatic drug-metabolizing enzymes, flumequine was administered to male CD-1 mice in the feed at doses equal to 0, 25, 50, 100, 400, or 800 mg/kg bw per day and to females at 0, 100, 400, or 800 mg/kg bw per day. ... Flumequine caused little or no induction of hepatic cytochrome P450-dependent drug-metabolizing enzymes or glucuronyltransferase when given at doses up to 800 mg/kg bw per day. ...
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 39: Flumequine (1997). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
To determine the plasma and urine levels of flumequine and its metabolite, 7-hydroxyflumequine, 28 healthy male subjects were given single and multiple oral doses of 400, 800 and 1200 mg flumequine. Results showed mean concentrations at 2 hr of 13.5, 23.8 and 31.9 mg/L, respectively. These levels were sustained up to 6 hr postdose. Following a single 800 mg dose, peak plasma levels of 14-25 mg/L occurred between 2.5 and 3.5 hr. The mean elimination half-life was 7.1 hr. In plasma only minimal levels of 7-hydroxyflumequine were found. Following 800 mg of flumequine four times a day, mean trough plasma levels of unchanged drug ranged from 21-23 mg/L. Mean peak concentrations were 41 mg/L at steady-state. The half-life following the last dose (8.5 hr) was not significantly different from the 7.1 hr half-life following the first dose. Substantial drug levels were present in the urine for 24 hr following single oral doses of 400, 800 and 1200 mg of flumequine. Urine levels of 7-hydroxyflumequine were generally higher than the parent compound. In the multiple dose study, the overnight concentration of flumequine always exceeded 50 mg/L, and the overnight concentration of 7-hydroxyflumequine always exceeded 80 mg/L.
Schuppan D et al; J Antimicrob Chemother 15 (3): 337-343 (1985)
... /In rats/ after administration of the 25 mg/kg bw oral dose ... the plasma half-life for flumequine was 5.25 hours.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
... /In male dogs/ after an oral dose of 25 mg/kg bw ... the disappearance of flumequine from the plasma appeared to follow multi-exponential kinetics with an initial half-life of about 75 minutes and a terminal beta-phase half-life of 6.5 hours.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 33: Flumequine (1994). Available from, as of October 13, 2015: https://www.inchem.org/pages/jecfa.html
... After IV and oral administration /in chickens/ (single-dose of 12 mg flumequine/kg bw ... elimination half-life and mean residence time of flumequine in plasma were 6.91 and 5.90 hr, respectively, after IV administration and 10.32 and 8.95 hr after oral administration. ...
PMID:1795097 Anadon A et al; Food Chem Toxicol 46 (2): 662-70 (2008)
To determine the plasma and urine levels of flumequine and its metabolite, 7-hydroxyflumequine, 28 healthy male subjects were given single and multiple oral doses of 400, 800 and 1200 mg flumequine. ... Following a single 800 mg dose, peak plasma levels of 14-25 mg/L occurred between 2.5 and 3.5 hr. The mean elimination half-life was 7.1 hr. ... Following 800 mg of flumequine four times a day ... the half-life following the last dose (8.5 hr) was not significantly different from the 7.1 hr half-life following the first dose.
Schuppan D et al; J Antimicrob Chemother 15 (3): 337-343 (1985)