
1. Fluni 1a Pharma
2. Flunibeta
3. Flunimerck
4. Fluninoc
5. Flunitrazepam Neuraxpharm
6. Flunitrazepam Ratiopharm
7. Flunitrazepam Teva
8. Flunitrazepam-neuraxpharm
9. Flunitrazepam-ratiopharm
10. Flunitrazepam-teva
11. Flunizep Von Ct
12. Fluridrazepam
13. Narcozep
14. Ro-5-4200
15. Ro54200
16. Rohipnol
17. Rohypnol
18. Von Ct, Flunizep
1. Rohypnol
2. 1622-62-4
3. Narcozep
4. Roipnol
5. Fluninoc
6. Flunipam
7. Flunitrazepamum
8. Flunitrazepamum [inn-latin]
9. Ro 5-4200
10. Silece
11. [3h]flunitrazepam
12. Ro-5-4200
13. Flunitrazepan
14. 5-(2-fluorophenyl)-1-methyl-7-nitro-3h-1,4-benzodiazepin-2-one
15. Ro54200
16. 5-(o-fluorophenyl)-1,3-dihydro-1-methyl-7-nitro-2h-1,4-benzodiazepin-2-one
17. 2h-1,4-benzodiazepin-2-one, 5-(2-fluorophenyl)-1,3-dihydro-1-methyl-7-nitro-
18. N05cd03
19. 1-methyl-7-nitro-5-(2-fluorophenyl)-3h-1,4-benzodiazepin-2(1h)-one
20. 1,3-dihydro-5-(o-fluorophenyl)-1-methyl-7-nitro-2h-1,4-benzodiazepin-2-one
21. Ro-54200
22. Primun
23. Chembl13280
24. Fluridrazepam
25. 5-(2-fluorophenyl)-1-methyl-7-nitro-1,3-dihydro-2h-1,4-benzodiazepin-2-one
26. 2h-1,4-benzodiazepin-2-one, 1,3-dihydro-5-(2-fluorophenyl)-1-methyl-7-nitro-
27. 620x0222fq
28. 5-(2-fluorophenyl)-1-methyl-7-nitro-2,3-dihydro-1h-1,4-benzodiazepin-2-one
29. Flunidazepam
30. Flunitrazepam [usan:inn:ban:jan]
31. Rohypnol (tn)
32. Ccris 5287
33. Hsdb 6960
34. Einecs 216-597-8
35. Brn 0702691
36. Hipnosedon
37. Flunita
38. Fluscand
39. Hypnodorm
40. Valsera
41. Hypnor
42. Primum
43. Dea No. 2763
44. Unii-620x0222fq
45. [3h]rohypnol
46. Flunitrazepam [mi]
47. 5-(2-fluorophenyl)-1,3-dihydro-1-methyl-7-nitro-2h-1,4-benzodiazepin-2-one
48. 5-(2-fluorophenyl)-1-methyl-7-nitro-1h-benzo[e][1,4]diazepin-2(3h)-one
49. Flunitrazepam [inn]
50. Flunitrazepam [jan]
51. Flunitrazepam [hsdb]
52. Flunitrazepam [usan]
53. Schembl44169
54. 5-24-04-00350 (beilstein Handbook Reference)
55. Mls003899224
56. Divk1c_000981
57. Flunitrazepam [mart.]
58. Flunitrazepam [who-dd]
59. Gtpl4193
60. Gtpl4360
61. Dtxsid7023065
62. Bdbm25878
63. Chebi:31622
64. Hms503e03
65. Kbio1_000981
66. Ninds_000981
67. Flunitrazepam (jp17/usan/inn)
68. Zinc3812994
69. Flunitrazepam [ep Monograph]
70. 5-(2-fluorophenyl)-1-methyl-7-nitro-3h-1,4-benzodiazepin-2(1h)-one
71. Nsc708829
72. Flunitrazepam 0.1 Mg/ml In Methanol
73. Flunitrazepam 1.0 Mg/ml In Methanol
74. Akos005066009
75. Db01544
76. Nsc-708829
77. Idi1_000981
78. Smr000058981
79. Db-043520
80. D01230
81. Q62947
82. 622f624
83. A810363
84. W-107962
85. Flunitrazepam Solution, Drug Standard, 1.0 Mg/ml In Methanol
86. Flunitrazepam, European Pharmacopoeia (ep) Reference Standard
87. Flunitrazepam-13c6, 100 Mug/ml In Methanol, Certified Reference Material
88. (e)-5-(2-fluorophenyl)-1-methyl-7-nitro-1h-benzo[e][1,4]diazepin-2(3h)-one
89. 1,3-dihydro-5-(2-fluorophenyl)-1-methyl-7-nitro-2h-1,4-benzodiazepin-2-one
90. 5-(2-fluorophenyl)-1-methyl-7-nitro-1,3-dihydro-2h-1,4-benzodiazepin-2-one #
91. 5-(2-fluorophenyl)-1-methyl-7-nitro-1,3-dihydrobenzo[e][1,4]diazepin-2-one
92. Flunitrazepam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
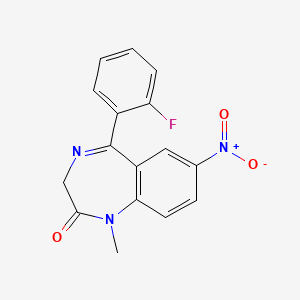
| Molecular Weight | 313.28 g/mol |
|---|---|
| Molecular Formula | C16H12FN3O3 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 313.08626942 g/mol |
| Monoisotopic Mass | 313.08626942 g/mol |
| Topological Polar Surface Area | 78.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Anxiety Agents; GABA Modulators
National Library of Medicine's Medical Subject Headings online file (MeSH, 2013)
Flunitrazepam is a short-acting benzodiazepine with general properties similar to those of diazepam. It is used in the short-term management of insomnia, as a premedication in surgical procedures, and for induction of anestesia.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
Flunitrazepam is tasteless and odorless and has been misused to incapacitate the victim and produce amnesia in sexual assaults and drug-facilitated rape ('date rape'). A 1-mg dose may produce impairment for 8-12 hrs. Some manufacturers have incorporated a blue dye into flunitrazepam tablets to increase visibility when placed into drinks but caution is still necessary as it has been reported that blue tropical drinks and punches are being used to overcome this.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
A WHO review concluded that flunitrazepam had a moderate abuse potential that might be higher than that of other benzodiazepines. It was reported that there was current evidence of widespread abuse of flunitrazepam among drug abusers, particularly among those who used opioids or cocaine.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
Of 43 patients given a single intravenous dose of flunitrazepam 1 to 2 mg, two had local thrombosis 7 to 10 days later. The incidence was lower than in those given diazepam (in solution). However, there was little difference in the incidence of local reactions after intravenous use of flunitrazepam and diazepam in another study.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
...accumulation in the milk might occur after repeated use.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
For short-term treatment of severe insomnias, that are not responsive to other hypnotics.
Flunitrazepam is a powerful hypnotic drug that is a benzodiazepine derivative. It has powerful hypnotic, sedative, anxiolytic, and skeletal muscle relaxant properties. The drug is sometimes used as a date rape drug. In the United States, the drug has not been approved by the Food and Drug Administration for medical use, and is considered to be an illegal drug. It has however been approved in the United Kingdom and other countries.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
N05CD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CD - Benzodiazepine derivatives
N05CD03 - Flunitrazepam
Absorption
50% (suppository) and 64-77% (oral)
... Flunitrazepam crosses the placenta slowly. About 12 hr after a 1 mg oral dose cord:maternal blood ratios in early and late pregnancy were about 0.5 and 0.22, respectively. Amniotic fluid:maternal serum ratios were in the 0.02-0.07 range in both cases. accumulation in the fetus may occur after repeated doses.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Nineth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2011, p. 580
Flunitrazepam is excreted into breast milk. Following a single 2 mg oral dose in five patients, mean milk:plasma ratios at 11, 15, 27, and 39 hr were 0.61, 0.68, 0.9, and 0.75, respectively.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Nineth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2011, p. 580
... The objective of this paper was to study elimination of flunitrazepam and 7-aminoflunitrazepam in urine collected from 10 healthy volunteers who received a single 2 mg oral dose of Rohypnol, to determine how long after drug administration 7-aminoflunitrazepam can be detected. A highly sensitive NCI-GC-MS method for the simultaneous quantitation of flunitrazepam (LOQ 100 pg/mL) and 7-aminoflunitrazepam (LOQ 10 pg/mL) in urine was developed. All samples were screened for benzodiazepines using optimized micro-plate enzyme immunoassay. The highest concns of 7-aminoflunitrazepam (70-518 ng/mL) and flunitrazepam (0.7-2.8 ng/mL) in urine were observed 6 hr after drug administration in 9 subjects and after 24 hr in one subject. In 6 subjects 7-aminoflunitrazepam was detected up to 14 days after flunitrazepam admin, in one subject up to 21 days and in 3 subjects up to 28 days. In urine samples collected from 6 volunteers, flunitrazepam was detected 3 days after Rohypnol intake, in 3 subjects 24 hr, and in one subject 5 days later. Benzodiazepine micro-plate enzyme immunoassay kit allowed the detection of flunitrazepam and metabolities 5 to 21 days after drug administration.
PMID:11005178 Negrusz A et al; J Forensic Sci 45 (5): 1031-1040 (2000)
To study the pharmacokinetics of flunitrazepam (used for sedation in neonates and infants), to determine the influence of both gestational and postnatal age on the pharmacokinetic parameters, and to analyze the relationship between the hemodynamic parameters and flunitrazepam plasma concentration. Flunitrazepam was infused for 20 minutes as a single dose (0.2 mg x kg(-1)) and as multiple doses (0.1 mg x kg(-1)). Six to eight 1-mL blood samples were collected per patient. Flunitrazepam plasma concentration was measured by gas chromatography-mass spectrometry. Thirty-one patients (25 neonates and six infants) were included in the study. Only three of them received multiple doses. After the single dose (n = 28), half-life was 22.6 +/- 7.3 hours, clearance was 0.15 +/- 0.14 L x kg x h(-1), and volume of distribution was 4.6 +/- 4.1 L x kg(-1) (mean +/- SD). Plasma clearance and volume of distribution significantly increased with postnatal age (P < .05), but no pharmacokinetic parameter varied significantly with gestational age. Diastolic blood pressure significantly decreased with increasing flunitrazepam plasma concentrations (P < .05). Postnatal age but not gestational age influenced flunitrazepam pharmacokinetic parameters in neonates and infants. Diastolic blood pressure was inversely correlated to flunitrazepam plasma concentration.
PMID:10460067 Pariente-Khayat A et al; Clin Pharmacol Ther 66 (2): 136-9 (1999)
Hepatic.
/The authors/ have identified CYP2C19 & CYP3A4 as the principal cytochrome P450s involved in the metab of flunitrazepam to its major metabolites desmethylflunitrazepam & 3-hydroxyflunitrazepam. Human CYP2C19 & CYP3A4 mediated the formation of desmethylflunitrazepam with Km values of 11.1 & 108 microM, respectively, & 3-hydroxyflunitrazepam with Km values of 642 & 34.0 microM, respectively. In human liver microsomes (n=4) formation of both metabolites followed biphasic kinetics. Desmethylflunitrazepam formation was inhibited 31% by S-mephenytoin & 78% by ketoconazole, suggesting involvement of both CYP2C19 & CYP3A4. Formation of 3-hydroxyflunitrazepam was also significantly inhibited by ketoconazole (94%) & S-mephenytoin (18%). In support of these chemical inhibition data, antibodies directed against CYP2C19 & CYP3A4 selectively inhibited formation of desmethylflunitrazepam by 26 & 45%, respectively, while anti-CYP3A4 antibodies reduced 3-hydroxyflunitrazepam formation by 80%. Our data also suggest that CYP1A2, -2B6, -2C8, -2C9, -2D6, & -2E1 are not involved in either of these metabolic pathways. /The authors/ estimate that the relative contributions of CYP2C19 & CYP3A4 to the formation of desmethylflunitrazepam in vivo are 63 & 37%, respectively, at therapeutic flunitrazepam concns (0.03 microM). /The authors/ conclude that the polymorphic enzyme CYP2C19 importantly mediates flunitrazepam demethylation, which may alter the efficacy & safety of the drug, while CYP3A4 catalyzes the formation of 3-hydroxyflunitrazepam.
PMID:11259331 Kilicarslan T et al; Drug Metab Dispos 29 (4 Pt 1): 460-465 (2001)
The aims were to examine the kinetics of the oxidative metabolism of flunitrazepam in vitro when flunitrazepam was dissolved in dimethylformamide and acetonitrile, and to determine which cytochrome P450 isoform(s) are involved. The kinetics of the formations of 3'-hydroxyflunitrazepam and desmethyl-flunitrazepam were non-linear and best estimated using the Hill equation. Inhibition of their formation was studied using specific chemical inhibitors, expressed enzyme systems and specific antibodies. Ks, Vmax, Clmax and n (slope factor) for the formation of 3'-hydroxyflunitrazepam and desmethylflunitrazepam had ranges of 165-338 and 179-391 microM, 22-81 and 3-10 nmol x mg protein(-1) x h(-1), 6-17 and 0.9-1.9 microl x mg protein(-1) x h(-1), and 2.3-3.6 and 1.6-2.6 respectively when dimethylformamide was the organic solvent. When acetonitrile was the solvent, Ks, Vmax, Clmax and n (slope factor) for the formation of 3'-hydroxyflunitrazepam and desmethylflunitrazepam had ranges of 173-231 and 74-597 microM, 35-198 and 2.7-48 nmol.mg protein(-1) x h(-1), 1347 and 0.7-6.3 microl.mg protein(-1) x h(-1), and 1.5-3.6 and 1.1-2.7 respectively. CYP2C19, CYP3A4 and CYP1A2 mediated the formation of both 3'-hydroxyflunitrazepam and desmethylflunitrazepam. Investigators need carefully to consider the choice of organic solvent to avoid false CYP identification.
PMID:10574680 Coller JK et al; Xenobiotica 29 (10): 973-86 (1999)
In recent years, there has been a notable increase in the number of reports on drug-facilitated sexual assault. Benzodiazepines are the most common so-called "date-rape" drugs, with flunitrazepam (Rohypnol) being one of the most frequently mentioned. The aim of this study was to determine whether flunitrazepam & its major metabolite 7-aminoflunitrazepam could be detected in hair collected from 10 healthy volunteers after receiving a single 2 mg dose of Rohypnol using solid phase extraction & NCI-GC-MS. Such data would be of great importance to law enforcement agencies trying to determine the best time interval for hair collection from a victim of drug-facilitated sexual assault in order to reveal drug use. Ten healthy volunteers (8 women & 2 men, 21 to 49 yr old) participated in the study. The following hair samples were collected from each volunteer: one before flunitrazepam admin, & 1, 3, 5, 14, 21, & 28 days after. In 5 volunteers, 7-aminoflunitrazepam was detected 24 hr after flunitrazepam admin & remained in hair throughout the entire 28-day study period (0.6-8.0 pg/mg). In 2 cases, 7-aminoflunitrazepam appeared in hair 21 days after drug intake (0.5-2.7 pg/mg), & in two subjects 14 days later (0.5-5.4 pg/mg). In one volunteer, 7-aminoflunitrazepam was detected on day 14 & 21 but concns were below the quantitation limit. Flunitrazepam was detected in some samples but all concns were below the quantitation limit (0.5-2.3 pg/mg).
PMID:11569557 Negrusz A et al; J Forensic Sci 46 (5): 1143-1151 (2001)
Flunitrazepam has known human metabolites that include 3-hydroxyflunitrazepam and desmethylflunitrazepam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
18-26 hours
The elimination half-life of flunitrazepam is reported to be between 16 and 35 hrs.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 996
Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.