
1. Acetonide, Fluocinolone
2. Acetonide, Fluortriamcinolone
3. Alvadermo
4. Capex
5. Co Fluocin
6. Co-fluocin
7. Cortiespec
8. Derma Smooth Fs
9. Derma-smooth Fs
10. Flucinar
11. Fluocid
12. Fluodermo
13. Fluonid
14. Fluortriamcinolone Acetonide
15. Fluotrex
16. Flurosyn
17. Flusolgen
18. Gelidina
19. Jellin
20. Jellisoft
21. Synalar
22. Synalar Hp
23. Synalar-hp
24. Synamol
25. Synemol
1. 67-73-2
2. Synalar
3. Fluonid
4. Flucinar
5. Synandone
6. Jellin
7. Synamol
8. Synemol
9. Coriphate
10. Fluovitif
11. Flupollon
12. Percutina
13. Synandrone
14. Dermalar
15. Localyn
16. Omniderm
17. Radiocin
18. Retisert
19. Sinalar
20. Synotic
21. Tefunote
22. Synsac
23. Fluotrex
24. Fluocet
25. Derma-smoothe/fs
26. Synalar-hp
27. Localyn Syntex
28. Cortiplastol
29. Iluvien
30. Prodermin
31. Capex
32. Fs Shampoo
33. Fluocinolone 16,17-acetonide
34. 6alpha-fluorotriamcinolone Acetonide
35. Fluocinoloni Acetonidum
36. Rs-1401 At
37. Acetonide De Fluocinolone
38. Component Of Neo-synalar
39. Fluocinolone (acetonide)
40. Oto-synalar
41. Flucort-n
42. Dermatin (steroid)
43. 6alpha,9alpha-difluoro-16alpha-hydroxyprednisolone 16,17-acetonide
44. Nsc-92339
45. Flucinolone Acetonide
46. Fluocinolide (acetate)
47. 0cd5fd6s2m
48. Mls000028545
49. Fluocinolonacetonidum
50. Chebi:31623
51. Fluocinolone Acetonide (flucort-n)
52. Nsc92339
53. Smr000058329
54. Mfcd00010525
55. Fluocinolone Acetonide [dcit]
56. Dsstox_cid_20674
57. Dsstox_rid_79533
58. Dsstox_gsid_40674
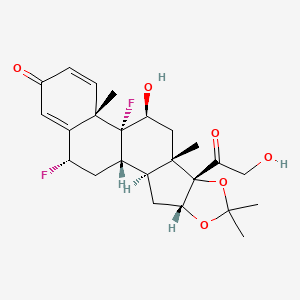
59. C24h30f2o6
60. Fluocinoloni Acetonidum [inn-latin]
61. Acetonida De Fluocinolona
62. Rs 1401at
63. 6.alpha.-fluorotriamcinolone Acetonide
64. Cas-67-73-2
65. Retisert (tn)
66. Fluocet (tn)
67. Synalar (tn)
68. Acetonide De Fluocinolone [inn-french]
69. Acetonida De Fluocinolona [inn-spanish]
70. Ccris 3250
71. Hsdb 3083
72. 6.alpha.,9-difluoro
73. Einecs 200-668-5
74. Nsc 92339
75. Unii-0cd5fd6s2m
76. Dermatin
77. Dermotic
78. Fluzon [steroid]
79. Ncgc00021301-04
80. (6alpha,9alpha,11beta,16beta)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione
81. 6-alpha,9-alpha-difluoro-16-alpha-hydroxyprednisolone 16,17-acetonide
82. 6.alpha.,9-difluoro-11.beta.,16.alpha.,17,21-tetrahydroxypregna-1,4-diene-3,20-dione, Cyclic 16,17-acetal With Acetone
83. Fluocinoloneacetonide
84. Fluocinolone-acetonide
85. Yutiq
86. 6alpha,9-difluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione, Cyclic 16,17-acetal With Acetone
87. Pregna-1,4-diene-3,20-dione, 6,9-difluoro-11,21-dihydroxy-16,17-((1-methylethylidene)bis(oxy))-, (6alpha,11beta,16alpha)-
88. Pregna-1,4-diene-3,20-dione, 6alpha,9-difluoro-11beta,16alpha,17,21-tetrahydroxy-, Cyclic 16,17-acetal With Acetone
89. Fluocinolone Acetonide Intravitreal Implant
90. Acetonido De Fluocinolona
91. 6.alpha.,17-acetonide
92. Opera_id_1295
93. F0657
94. Fluocinolone Acetonide [usan:usp:inn:jan]
95. Chembl989
96. Schembl4795
97. Fluocinolone Acetonide - Bp
98. 6alpha,9alpha-difluoro-11beta,16alpha,17alpha,21-tetrahydroxy-1,4-pregnadiene-3,20-dione 16,17-acetonide
99. Mls001076276
100. Gtpl7077
101. Dtxsid0040674
102. Hms2090i14
103. Hms2230d13
104. Hms3259g12
105. Hms3715j11
106. Fluocinolone Acetonide [mi]
107. Hy-b0415
108. Zinc3977981
109. Fluocinolone Acetonide [inn]
110. Fluocinolone Acetonide [jan]
111. Tox21_110869
112. Tox21_302364
113. Ac-429
114. Ac1069
115. Fluocinolone Acetonide [hsdb]
116. Fluocinolone Acetonide [usan]
117. S2470
118. Fluocinolone Acetonide [vandf]
119. Akos015963144
120. Fluocinolone Acetonide [mart.]
121. Tox21_110869_1
122. Ccg-221165
123. Db00591
124. Fluocinolone Acetonide [usp-rs]
125. Fluocinolone Acetonide [who-dd]
126. Nc00562
127. Fluocinolone Acetonide (jp17/usp/inn)
128. Ncgc00021301-06
129. Ncgc00255654-01
130. (4as,4br,5s,6as,6bs,9ar,10as,10bs,12s)-4b,12-difluoro-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2h-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one
131. (4as,4br,5s,6as,6bs,9ar,10as,10bs,12s)-4b,12-difluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2h-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one
132. 4b,12-difluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2h-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one
133. As-13690
134. Bf176452
135. Nci60_042042
136. Fluocinolone Acetonide [green Book]
137. Fluocinolone Acetonide, Analytical Standard
138. Fluocinolone Acetonide [orange Book]
139. Fluocinolone Acetonide [ep Monograph]
140. Fluocinolone Acetonide [usp Impurity]
141. Fluocinolone Acetonide For System Suitability
142. Fluocinolone Acetonide [usp Monograph]
143. Otovel Component Fluocinolone Acetonide
144. D01825
145. Iluvien Component Fluocinolone Acetonide
146. Ab00383017-10
147. Ab00383017_11
148. Fluocinolone Acetonide Component Of Otovel
149. Otovel Component Of Fluocinolone Acetonide
150. Tri-luma Component Fluocinolone Acetonide
151. 010f525
152. A853087
153. Fluocinolone Acetonide 100 Microg/ml In Methanol
154. Fluocinolone Acetonide Component Of Iluvien
155. Neo-synalar Component Fluocinolone Acetonide
156. Q924467
157. Sr-01000000109
158. Fluocinolone Acetonide Component Of Tri-luma
159. Sr-01000000109-2
160. W-104704
161. Brd-k94353609-001-21-6
162. Difluoro-hydroxy-(2-hydroxyacetyl)-tetramethyl-[?]one
163. Fluocinolone Acetonide Component Of Neo-synalar
164. 6.alpha., 9.alpha.-difluoro-16.alpha.-hydroxyprednisolone 16,17-acetonide
165. Fluocinolone Acetonide, European Pharmacopoeia (ep) Reference Standard
166. Fluocinolone Acetonide, United States Pharmacopeia (usp) Reference Standard
167. Fluocinolone Acetonide For System Suitability, European Pharmacopoeia (ep) Reference Standard
168. Fluocinolone Acetonide, Pharmaceutical Secondary Standard; Certified Reference Material
169. Wln: T F5 E5 B666 Go Io Rv Ahtttt&j A Bf Cq E Fviq H H Of -a&bho -b&acef
170. (1s,2s,4r,8s,9s,11s,12r,13s,19s)-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icosa-14,17-dien-16-one
171. (2s,6as,6br,7s,8as,8bs,11ar,12as,12bs)-2,6b-difluoro-7-hydroxy-8b-(2-hydroxyacetyl)-6a,8a,10,10-tetramethyl-6a,6b,7,8,8a,8b,11a,12,12a,12b-decahydro-1h-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-4(2h)-one
172. (6a,11b,16a)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione
173. (6alpha,11beta,16alpha)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione
174. 6.alpha.,16.alpha.,17,21-tetrahydroxypregna-1,4-diene-3,20-dione, Cyclic 16,17-acetal With Acetone
175. Fluocinolone Acetonide For Peak Identification, European Pharmacopoeia (ep) Reference Standard
176. Pregna-1,20-dione, 6,9-difluoro-11,12-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (6.alpha.,11.beta.,16.alpha.)-
177. Pregna-1,20-dione, 6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)-, (6.alpha.,11.beta.,16.alpha.)-
178. Pregna-1,20-dione, 6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (6.alpha.,11.beta.,16.alpha.)-
179. Pregna-1,20-dione, 6.alpha.,9-difluoro-11.beta.,16.alpha.,17,21-tetrahydroxy-, Cyclic 16,17-acetal With Acetone
180. Pregna-1,4-diene-3,20-dione, 6,9-difluoro-11,21-dihydroxy-16,17-((1-methylethylidene)bis(oxy))-, (6.alpha.,11.beta.,16.alpha.)-
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C24H30F2O6 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 452.20104500 g/mol |
| Monoisotopic Mass | 452.20104500 g/mol |
| Topological Polar Surface Area | 93.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 960 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Fluocinolone acetonide |
| PubMed Health | Fluocinolone Acetonide (Into the ear) |
| Drug Classes | Otic Agent |
| Drug Label | Fluocinolone Acetonide 0.01 % Topical Oil contains fluocinolone acetonide {(6, 11, 16)-6,9-difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone}, a synthetic corticosteroid f... |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Ointment; Cream; Oil; Oil/drops; Solution |
| Route | Topical; Otic |
| Strength | 0.025%; 0.01% |
| Market Status | Prescription |
| Company | Taro; Fougera; Identi Pharms; G And W Labs; Perrigo Israel |
| 2 of 10 | |
|---|---|
| Drug Name | Iluvien |
| PubMed Health | Fluocinolone |
| Drug Classes | Corticosteroid, Intermediate, Fluocinolone, Otic Agent |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Insert |
| Route | Intravitreal |
| Strength | 0.19mg |
| Market Status | Prescription |
| Company | Alimera Sciences |
| 3 of 10 | |
|---|---|
| Drug Name | Neo-synalar |
| PubMed Health | Fluocinolone |
| Drug Classes | Corticosteroid, Intermediate, Fluocinolone, Otic Agent |
| Drug Label | RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg is a sterile implant designed to release fluocinolone acetonide locally to the posterior segment of the eye at a nominal initial rate of 0.6 g/day, decreasing over the first month to... |
| Active Ingredient | Fluocinolone acetonide; neomycin sulfate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.025%; eq 3.5mg base/gm |
| Market Status | Prescription |
| Company | Medimetriks Pharms |
| 4 of 10 | |
|---|---|
| Drug Name | Retisert |
| Drug Label | SYNALAR (fluocinolone acetonide) Topical Solution 0.01% is intended for topical administration. The active component is the corticosteroid fluocinolone acetonide, which has the chemical name pregna-1,4-diene-3,20-dione,6,9-difluoro-11,21-dihydroxy-... |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Implant |
| Route | Intravitreal |
| Strength | 0.59mg |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 5 of 10 | |
|---|---|
| Drug Name | Synalar |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Ointment; Cream; Solution |
| Route | Topical |
| Strength | 0.025%; 0.01% |
| Market Status | Prescription |
| Company | Medimetriks Pharms |
| 6 of 10 | |
|---|---|
| Drug Name | Fluocinolone acetonide |
| PubMed Health | Fluocinolone Acetonide (Into the ear) |
| Drug Classes | Otic Agent |
| Drug Label | Fluocinolone Acetonide 0.01 % Topical Oil contains fluocinolone acetonide {(6, 11, 16)-6,9-difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone}, a synthetic corticosteroid f... |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Ointment; Cream; Oil; Oil/drops; Solution |
| Route | Topical; Otic |
| Strength | 0.025%; 0.01% |
| Market Status | Prescription |
| Company | Taro; Fougera; Identi Pharms; G And W Labs; Perrigo Israel |
| 7 of 10 | |
|---|---|
| Drug Name | Iluvien |
| PubMed Health | Fluocinolone |
| Drug Classes | Corticosteroid, Intermediate, Fluocinolone, Otic Agent |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Insert |
| Route | Intravitreal |
| Strength | 0.19mg |
| Market Status | Prescription |
| Company | Alimera Sciences |
| 8 of 10 | |
|---|---|
| Drug Name | Neo-synalar |
| PubMed Health | Fluocinolone |
| Drug Classes | Corticosteroid, Intermediate, Fluocinolone, Otic Agent |
| Drug Label | RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg is a sterile implant designed to release fluocinolone acetonide locally to the posterior segment of the eye at a nominal initial rate of 0.6 g/day, decreasing over the first month to... |
| Active Ingredient | Fluocinolone acetonide; neomycin sulfate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.025%; eq 3.5mg base/gm |
| Market Status | Prescription |
| Company | Medimetriks Pharms |
| 9 of 10 | |
|---|---|
| Drug Name | Retisert |
| Drug Label | SYNALAR (fluocinolone acetonide) Topical Solution 0.01% is intended for topical administration. The active component is the corticosteroid fluocinolone acetonide, which has the chemical name pregna-1,4-diene-3,20-dione,6,9-difluoro-11,21-dihydroxy-... |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Implant |
| Route | Intravitreal |
| Strength | 0.59mg |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 10 of 10 | |
|---|---|
| Drug Name | Synalar |
| Active Ingredient | Fluocinolone acetonide |
| Dosage Form | Ointment; Cream; Solution |
| Route | Topical |
| Strength | 0.025%; 0.01% |
| Market Status | Prescription |
| Company | Medimetriks Pharms |
Glucocorticoids, Synthetic; Glucocorticoids, Topical
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
WITH EXCEPTION OF SUBSTITUTION THERAPY, USE OF CORTICOSTEROIDS & THEIR CONGENERS IN DISEASE IS EMPIRICAL. ...FOR ANY DISEASE, IN ANY PT, APPROPRIATE DOSE TO ACHIEVE GIVEN THERAPEUTIC EFFECT MUST BE DETERMINED BY TRIAL & ERROR & MUST BE REEVALUATED FROM TIME TO TIME AS STAGE & ACTIVITY OF DISEASE ALTER... /CORTICOSTEROID/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1497
GLUCOCORTICOID WITH POTENT ANTI-INFLAMMATORY & METABOLIC ACTIONS & NEGLIGIBLE MINERALOCORTICOID ACTIONS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 892
IT IS EMPLOYED TOPICALLY IN TREATMENT OF VARIOUS DERMATOSES. IN RESISTANT NUMMULAR DERMATITIS, PSORIASIS, OR CHRONIC NEURODERMATITIS USUALLY USED UNDER OCCLUSIVE DRESSINGS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 892
For more Therapeutic Uses (Complete) data for FLUOCINOLONE ACETONIDE (7 total), please visit the HSDB record page.
...AS CORTICOSTEROID THERAPY IS PROLONGED OVER PERIODS OF MO, & TO EXTENT THAT DOSE EXCEEDS EQUIV OF SUBSTITUTION THERAPY, INCIDENCE OF DISABLING & POTENTIALLY LETHAL EFFECTS INCR; EXCEPT IN ADRENAL INSUFFICIENCY, ADMIN...IS NEITHER ETIOLOGICAL OR CURATIVE THERAPY BUT ONLY PALLIATIVE...ANTI-INFLAMMATORY... /CORTICOSTEROIDS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1497
EVEN IN INSTANCES IN WHICH NEARLY WHOLE BODY HAS BEEN COVERED BY CREAM CONTAINING CORTICOID, EVIDENCES OR SYSTEMIC SIDE EFFECTS ARE RARE. ... TOPICAL FLUOCINOLONE IS CONTRAINDICATED IN PRESENCE OF TUBERCULOSIS, FUNGAL INFECTIONS, & MOST VIRAL LESIONS OF SKIN (VACCINIA, VARICELLA, HERPES SIMPLEX, ETC).
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 892
...CAUTION SHOULD BE EXERCISED IF FLUORINATED PREPN ARE USED ON FACE OR OTHER COSMETICALLY IMPORTANT AREAS, SINCE PARADOXICAL SKIN ERUPTIONS MAY OCCUR WITH LONG-TERM USE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 518
VET: SOLN IN PROPYLENE GLYCOL USUALLY PRODUCES FAR MORE THAN "TRANSIENT STINGING SENSATION" DESCRIBED BY MFR & SHOULD BE AVOIDED ESP ON RAW OR DENUDED AREAS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 224
For more Drug Warnings (Complete) data for FLUOCINOLONE ACETONIDE (7 total), please visit the HSDB record page.
Fluocinolone acetonide has been used extensively in different medical areas. -In dermatology, it is extensively used for the relief of inflammatory dermatosis, dermatitis, psoriasis, hypertrophic tissues, keloid tissues and atopic dermatitis. -It has been used in shampoo products as a low to medium potency corticosteroid for the treatment of seborrheic dermatitis of the scalp. -In ear drops, it is used as a low to medium potency corticosteroid for the treatment of chronic eczematous external otitis in adults and pediatric patients 2 years and older. -As an intravitreal implant, it is indicated for the treatment of diabetic macular edema with patients that have been previously treated with a course of corticosteroids and no clinically significant rise in intraocular pressure. -Fluocinolone acetonide was announced on October 15, 2018 to be FDA approved for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye. -Some reports have indicated the use of fluocinolone acetonide as a vasoprotective agent and for its use in the treatment of first-degree hemorrhoids.
FDA Label
Treatment of dermatitis and eczema
Treatment of diabetic macular oedema
Treatment of chronic non-infectious uveitis
Fluocinolone acetonide is a synthetic anti-inflammatory corticosteroid and thus, the effect of its interaction with the body produces vasoconstriction and suppression of membrane permeability, mitotic activity, immune response and release of inflammatory mediators. For its ophthalmic indications, fluocinolone acetonide is administered as intravitreal micro-insert. This preparation was observed in clinical trials to reduce the recurrence of uveitis flares by 2 fold when compared with the non treated patients even after six months after initial administration. As well the intraocular pressure seemed to increase slightly with the presence of the fluocinolone implant but it is important to monitor intraocular pressure.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA10 - Fluocinolone acetonide
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC04 - Fluocinolone acetonide
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA15 - Fluocinolone acetonide
S - Sensory organs
S02 - Otologicals
S02B - Corticosteroids
S02BA - Corticosteroids
S02BA08 - Fluocinolone acetonide
Absorption
When administered as an eye implant, fluocinolone acetonide presents a sustained delivery for even 12 months in which there can be observed a sustained release. The concentration of fluocinolone acetonide are generally higher in the vitreous and retina with a little dispersion to the aqueous humor. There are reports indicating that topical administration of fluocinolone acetonide produces a percutaneous absorption which is determined by the vehicle, integrity of the epidermal barrier and the use of occlusive dressing. Independently of the route of administration, the systemic absorption of fluocinolone acetonide is below 0.1 ng/ml which indicates that the systemic distribution is very minimal and the effect of fluocinolone is mainly local.
Route of Elimination
Fluocinolone acetonide is mainly excreted by the kidneys. It is important to mention that the systemically absorbed dose is very minimal.
Volume of Distribution
This pharmacokinetic parameter is not relevant as the systemic absorption of fluocinolone acetonide is very minimal.
Clearance
This pharmacokinetic parameter is not relevant as the systemic absorption of fluocinolone acetonide is very minimal and the concentration in urine is lower than the minimum quantitation limit.
METABOLISM OF CORTICOSTEROIDS IS GREATLY SLOWED BY INTRODUCTION OF THE 1,2 DOUBLE BOND OR A FLUORINE ATOM INTO MOLECULE, & HALF-LIFE IS CORRESPONDINGLY PROLONGED. /CORTICOSTEROIDS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1489
(14)C WAS RAPIDLY & MAINLY EXCRETED IN 48-HR FECES (90%), VIA BILE (70% IN 24 HR), & IN URINE (8%). AFTER DERMAL APPLICATION OF CREAM CONTAINING [(14)C]FLUOCINOLONE ACETONIDE TO MICE, GREATER THAN 7% OF (14)C WAS ABSORBED.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 130
...WITHIN 1 HR OF SC DOSE OF [(14)C]FLUOCINOLONE ACETONIDE TO MICE, LARGE AMT...PRESENT IN LIVER & INJECTION SITE, LOWER AMT IN PANCREAS, KIDNEYS, SALIVARY GLANDS, MYOCARDIUM, PITUITARY, & LACRIMAL GLANDS. TISSUE (14)C DECR FAIRLY RAPIDLY DURING 24 HR, EXCEPT IN LIVER & INTESTINES, BECAUSE OF BILIARY SECRETION...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 130
PENETRATION OF...FLUOCINOLONE ACETONIDE...TOPICALLY APPLIED, THROUGH HUMAN ABDOMINAL SKIN WAS OPTIMAL WHEN CONCN OF DRUGS WAS MAXIMAL WHILE MAINTAINING FAVORABLE PARTITION COEFFICIENT BETWEEN SKIN BARRIER & VEHICLE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 426
For more Absorption, Distribution and Excretion (Complete) data for FLUOCINOLONE ACETONIDE (6 total), please visit the HSDB record page.
Following absorption, fluocinolone acetonide metabolism is primarily hepatic. It is important to mention that the systemically absorbed dose is very minimal.
...IT IS GENERALLY ASSUMED THAT METAB OF /CORTISOL &/ ITS CONGENERS & SYNTH DERIV IS QUALITATIVELY SIMILAR. ...METABOLIZED PRINCIPALLY BY REDN OF RING A, REDN OF KETONE AT C 20, & CLEAVAGE OF SIDE CHAIN. METABOLITES ARE EXCRETED...AS GLUCURONIDES, SULFATES, & UNCONJUGATED COMPOUNDS. /CORTICOSTEROIDS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1489
The reported half-life of fluocinolone acetonide ranges between 1.3-1.7 hours.
Fluocinolone acetonide is a corticosteroid and thus, it can be inferred that it acts by inhibiting the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, collagen deposition, and scar formation. Some reports have indicated that fluocinolone acetonide presents a high binding affinity for the glucocorticoid receptor. After binding the receptor, the newly formed receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements in the promoter region of the target genes. This effect promotes the induction of phospholipase A2 inhibitory proteins (lipocortins). Through this mechanism of action, it is thought that fluocinolone induces mainly one of the lipocortins, annexin 1, which will later mediate the synthesis of inflammatory mediators such as prostaglandins and leukotrienes by inhibiting the release of arachidonic acid which is the precursor of all these inflammatory mediators. Hence, the induction of these proteins will prevent the release of arachidonic acid by phospholipase A2.
CORTICOSTEROIDS...ARE THOUGHT TO ACT BY CONTROLLING RATE OF SYNTH OF PROTEINS. ...CORTICOSTEROIDS REACT WITH RECEPTOR PROTEINS IN CYTOPLASM OF SENSITIVE CELLS TO FORM STEROID-RECEPTOR COMPLEX. ... STEROID HORMONES APPEAR TO STIMULATE TRANSCRIPTION & ULTIMATELY SYNTH OF SPECIFIC PROTEINS. /CORTICOSTEROIDS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1480
...CORTICOSTEROIDS...INFLUENCE CARBOHYDRATE, PROTEIN, FAT, & PURINE METABOLISM; ELECTROLYTE & WATER BALANCE; & FUNCTIONS OF CVS, KIDNEY, SKELETAL MUSCLE, NERVOUS SYSTEM, & OTHER ORGANS & TISSUES. ...CORTICOSTEROIDS ENDOW ORGANISM WITH CAPACITY TO RESIST MANY TYPES OF NOXIOUS STIMULI & ENVIRONMENTAL CHANGE. /CORTICOSTEROIDS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1478