


1. Veramyst
2. Avamys
3. 397864-44-7
4. Allermist
5. Furamist
6. Arnuity Ellipta
7. Ennhale
8. Gsk 685 698
9. Flonase Sensimist
10. Gw685698x
11. Gsk 685698
12. Gw-685698x
13. Js86977wnv
14. Chebi:74899
15. Gsk685968
16. Gsk-685968
17. Gw 685698x
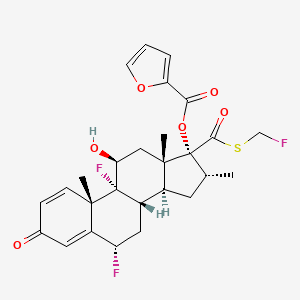
18. [(6s,8s,9r,10s,11s,13s,14s,16r,17r)-6,9-difluoro-17-(fluoromethylsulfanylcarbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Furan-2-carboxylate
19. Alisade
20. (6s,8s,9r,10s,11s,13s,14s,16r,17r)-6,9-difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl Furan-2-carboxylate
21. Unii-js86977wnv
22. Fluticasone Furoate [usan:inn]
23. Fluticasonum Furoas
24. Veramyst (tn)
25. Furoate De Fluticasone
26. Furoato De Fluticasona
27. Fluticasone Furancarboxylate
28. Chembl1676
29. Schembl140504
30. Flonase Sensimist Allergy Relief
31. Fluticasone Furoate [mi]
32. Gtpl10892
33. Fluticasone Furoate [inn]
34. Fluticasone Furoate [jan]
35. Gw685698
36. Dtxsid401024827
37. Fluticasone Furoate [usan]
38. Fluticasone Furoate [vandf]
39. Bcp18136
40. Fluticasone Furoate [mart.]
41. Zinc3992105
42. Bdbm50354851
43. Fluticasone Furoate [who-dd]
44. S6487
45. Fluticasone Furoate (jan/usan/inn)
46. Fluticasone Furoate [ema Epar]
47. Db08906
48. Fluticasone Furoate [orange Book]
49. Hy-15234
50. Avamys Pound>> Veramyst Pound>> Allermist
51. Gw-685698
52. Cs-0003822
53. Drosta-1,4-dien-17-yl Furan-2-carboxylate
54. D06315
55. E86983
56. Breo Ellipta Component Fluticasone Furoate
57. Arnuity Ellipta Component Fluticasone Furoate
58. Fluticasone Furoate Component Of Breo Ellipta
59. Q2166700
60. Trelegy Ellipta Component Fluticasone Furoate
61. Fluticasone Furoate Component Of Arnuity Ellipta
62. Fluticasone Furoate Component Of Trelegy Ellipta
63. (6.alpha.,11.beta.,16.alpha.,17.alpha.)-6,9-difluoro-17-(((fluoro-methyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furancarboxylate
64. (6.alpha.,11.beta.,16.alpha.,17.alpha.)-6,9-difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furoate
65. (6.alpha.,11.beta.,16.alpha.,17.alpha.)-6,9-difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl-2-furancarboxylate
66. (6alpha,11alpha,14beta,16alpha,17alpha)-6,9-difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11-hydroxy-16-methyl-3-oxoan
67. (6alpha,11alpha,14beta,16alpha,17alpha)-6,9-difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11-hydroxy-16-methyl-3-oxoan Drosta-1,4-dien-17-yl Furan-2-carboxylate
68. (6alpha,11alpha,14beta,16alpha,17alpha)-6,9-difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl Furan-2-carboxylate
69. (6alpha,11beta,16alpha,17alpha)-6,9-difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl-2-furancarboxylate
70. 6.alpha.,9-difluoro-17-(((fluoromethyl)sulfanyl)carbonyl)-11.beta.-hydroxy-16.alpha.-methyl-3-oxoandrosta-1,4-dien-17.alpha.-yl Furan-2-carboxylate
71. 6alpha,9-difluoro-17-(((fluoromethyl)sulfanyl)carbonyl)-11beta-hydroxy-16alpha-methyl-3-oxoandrosta-1,4-dien-17alpha-yl Furan-2-carboxylate
72. 6alpha,9-difluoro-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-11beta-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17alpha-yl 2-furoate
73. 911210-90-7
74. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-17-((2- Furanylcarbonyl)oxy)-11-hydroxy-16-methyl-3-oxo-, S-(fluoromethyl) Ester, (6alpha,11beta,16alpha,17alpha)-
75. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-17-((2- Furanylcarbonyl)oxy)-11-hydroxy-16-methyl-3-oxo-, S-(fluoromethyl) Ester, (6alpha,11beta,16alpha,17alpha)-
76. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-17-((2-furanylcarbonyl)oxy)-11-hydroxy-16-methyl-3-oxo-, S-(fluoromethyl) Ester, (6.alpha.,11.beta.,16.alpha.,17.alpha.)-
77. Gw6
| Molecular Weight | 538.6 g/mol |
|---|---|
| Molecular Formula | C27H29F3O6S |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 538.16369430 g/mol |
| Monoisotopic Mass | 538.16369430 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 1080 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Veramyst |
| PubMed Health | Fluticasone |
| Drug Classes | Anti-Inflammatory, Corticosteroid, Intermediate, Corticosteroid, Strong |
| Drug Label | Fluticasone furoate, the active component of VERAMYST Nasal Spray, is a synthetic fluorinated corticosteroid having the chemical name (6,11,16,17)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-... |
| Active Ingredient | Fluticasone furoate |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.0275mg/inh |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Veramyst |
| PubMed Health | Fluticasone |
| Drug Classes | Anti-Inflammatory, Corticosteroid, Intermediate, Corticosteroid, Strong |
| Drug Label | Fluticasone furoate, the active component of VERAMYST Nasal Spray, is a synthetic fluorinated corticosteroid having the chemical name (6,11,16,17)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-... |
| Active Ingredient | Fluticasone furoate |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.0275mg/inh |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Fluticasone furoate is indicated as an inhaler for the treatment and management of asthma by prophylaxis. The fluticasone furoate nasal spray is indicated for treating season and perennial allergic rhinitis.
FDA Label
Adults, adolescents (12 years and over) and children (6-11 years). Avamys is indicated for the treatment of the symptoms of allergic rhinitis.
Adults, adolescents (12 years and over) and children (6 - 11 years). Alisade is indicated for the treatment of the symptoms of allergic rhinitis.
Systemically, in vitro experiments show fluticasone furoate activates glucocorticoid receptors, inhibits nuclear factor kappa b, and inhibits lung eosinophilia in rats.
R01AD12
R01AD12
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AD - Corticosteroids
R01AD12 - Fluticasone furoate
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BA - Glucocorticoids
R03BA09 - Fluticasone furoate
Absorption
Intranasal exposure of fluticasone furoate results in patients swallowing a larger portion of the dose. However, absorption is poor and metabolism is high, therefore there is negligible systemic exposure with a nasal bioavailability of 0.50% and oral bioavialability of 1.26%. Inhaled bioavailability is 13.9%. A study of 24 healthy Caucasian males showed an inhaled bioavailability of 6.3-18.4%.
Route of Elimination
Fluticasone furoate is eliminated 90% in the feces and 1-2% in the urine.
Volume of Distribution
608L at steady state for intravenous administration of fluticasone furoate. Other reports suggest the mean volume of distribution at steady state is 661L. A study of 24 healthy Caucasian males showed a volume of distribution at steady state of 704L following intravenous administration.
Clearance
57.8L/h for fluticasone furoate. A study of 24 healthy Caucasian males showed a clearance of 71.8L/h following intravenous administration.
Fluticasone furoate is cleared from hepatic metabolism by cytochrome P450 3A4. Fluticasone furoate is hydrolysed at the FIVE-S-fluoromethyl carbothioate group, forming an inactive metabolite.
15.1 hours for intranasal fluticasone furoate and 24 hours for the inhaled formulation. A study of 24 healthy Caucasian males showed a half life of 13.6 hours following intravenous administration and 17.3-23.9 hours followed inhalation.
Fluticasone furoate works through an unknown mechanism to affect the action of various cell types and mediators of inflammation. In vitro experiments show fluticasone furoate activating glucocorticoid receptors, inhibiting nuclear factor kappa b, and inhibiting lung eosinophilia in rats.
