
1. Cutivate
2. Flixonase
3. Flixotide
4. Flonase
5. Flovent
6. Flovent Hfa
7. Fluticasone
8. Hfa, Flovent
9. Propionate, Fluticasone
1. 80474-14-2
2. Flovent
3. Cutivate
4. Flixotide
5. Flonase
6. Flixonase
7. Flovent Hfa
8. Flunase
9. Flusonal
10. Fluspiral
11. Flutide
12. Flutivate
13. Asmatil
14. Axotide
15. Brethal
16. Fluinol
17. Flovent Diskus 50
18. Flixotide Disks
19. Flixotide Disk
20. Flovent Diskus
21. Flovent Diskus 100
22. Flovent Diskus 250
23. Flixotide Inhaler
24. Cultivate
25. Flixonase Nasal Spray
26. Fluticasonpropionat Allen
27. Cci-18781
28. Xhance
29. Fluticasone (propionate)
30. Cci 18781
31. Armonair Respiclick
32. Fluticasone-17-propionate
33. O2gmz0lf5w
34. Atemur
35. Chebi:31441
36. Nsc-759889
37. Inalacor
38. Rinosone
39. Trialona
40. Ubizol
41. Zoflut
42. Fluticasone Propionate (flonase, Veramyst)
43. Mfcd00866007
44. Flonase Aq
45. Pf-00241939
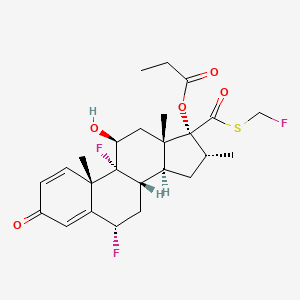
46. [(6s,8s,9r,10s,11s,13s,14s,16r,17r)-6,9-difluoro-17-(fluoromethylsulfanylcarbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Propanoate
47. Flovent-hfa
48. Fluticasone Propionate [usan]
49. Unii-o2gmz0lf5w
50. Fluxonal
51. Skyron
52. Flovent Rotadisk
53. Cutivate (tn)
54. Fluticasone Propionate [usan:usp]
55. Flonase (tn)
56. Flovent (tn)
57. Fluticasone-propionate
58. Armonair Respiclicktm
59. Flonase Allergy Relief
60. Fluticasone Impurity B
61. Prestwick0_000997
62. Prestwick1_000997
63. Prestwick2_000997
64. Prestwick3_000997
65. Cci18781
66. Schembl4068
67. Chembl1473
68. Bspbio_001093
69. Mls001424085
70. S-(fluoromethyl) 6alpha,9-difluoro-11beta,17-dihydroxy-16alpha-methyl-3-oxoandrosta-1,4-diene-17beta-carbothioate, 17-propionate
71. Fluticasone Propionate- Bio-x
72. Spbio_002984
73. Bpbio1_001203
74. Gtpl7080
75. Dtxsid8045511
76. Fluticasone Propionate (jan/usp)
77. Fn-25
78. Hms1571g15
79. Hms2051n19
80. Hms2098g15
81. Hms3413a19
82. Hms3677a19
83. Hms3715g15
84. Fluticasone Propionate [mi]
85. Amy38235
86. Hy-b0154
87. Ymb56612
88. Zinc3920027
89. Fluticasone Propionate [jan]
90. Ac-457
91. Bdbm50354849
92. S1992
93. Fluticasone Propionate [vandf]
94. Akos015895220
95. Fluticasone Propionate [mart.]
96. Ccg-100981
97. Cci-187881
98. Cs-1986
99. Db00588
100. Fluticasone Propionate [usp-rs]
101. Fluticasone Propionate [who-dd]
102. Ks-1173
103. Nc00231
104. Nsc 759889
105. Ncgc00179308-01
106. Ncgc00179308-05
107. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6alpha,11beta,16alpha,17alpha)-s-(fluoromethyl) Ester
108. Bf160362
109. Bf161262
110. Smr000469159
111. Fluticasone Propionate [orange Book]
112. Ab00513992
113. Fluticasone Propionate [ep Monograph]
114. Advair Component Fluticasone Propionate
115. Fluticasone Propionate [usp Monograph]
116. A51110
117. D01708
118. Dymista Component Fluticasone Propionate
119. Ab00513992-06
120. Ab00513992_08
121. Fluticasone Propionate - Micronised Pharma Grade
122. Fluticasone Propionate Component Of Advair
123. Fluticasone Propionate, >=98% (hplc), Powder
124. 474f142
125. Advair Hfa Component Fluticasone Propionate
126. An-584/43505443
127. Fluticasone Propionate 100 Microg/ml In Methanol
128. Fluticasone Propionate Component Of Dymista
129. Lipo-102 Component Fluticasone Propionate
130. Sr-01000763355
131. Q-101393
132. Q8564098
133. Sr-01000763355-3
134. Brd-k62310379-001-03-0
135. Fluticasone Propionate 100 Microg/ml In Acetonitrile
136. Fluticasone Propionate Component Of Advair Hfa
137. Airduo Respiclick Component Fluticasone Propionate
138. Fluticasone 17(2)-carbonylsulfenic Acid 17-propionate
139. Fluticasone Propionate Component Of Airduo Respiclick
140. Fluticasone Propionate, European Pharmacopoeia (ep) Reference Standard
141. Fluticasone Propionate, United States Pharmacopeia (usp) Reference Standard
142. Fluticasone Propionate, Pharmaceutical Secondary Standard; Certified Reference Material
143. (1r,2s,8s,10s,11s,13r,14r,15s,17s)-1,8-difluoro-14-{[(fluoromethyl)sulfanyl]carbonyl}-17-hydroxy-2,13,15-trimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl Propanoate
144. (6?,11?,16?,17?)-6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic Acid Fluoromethyl Ester
145. 6alpha,9-difluoro-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-11beta-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17alpha-yl Propanoate
146. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6.alpha.,11.beta.,16.alpha.,17.alpha.)-s-(fluoromethyl) Ester
147. Androsta-1,4-diene-17-carbothioic Acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-,s-(fluoromethyl) Ester, (6.alpha.,11.beta.,16.alpha.,17.alpha.)-
148. Fluticasone Propionate For Impurity C Identification, Europepharmacopoeia (ep) Reference Standard
149. Fluticasone Propionate For Impurity G Identification, Europepharmacopoeia (ep) Reference Standard
150. S-fluoromethyl 6.alpha., 9.alpha.-difluoro-11.beta.-hydroxy-16.alpha.-methyl-3-oxo-17.alpha.-propionyloxyandrosta-1,4-diene-17.beta-. Carbothioate
| Molecular Weight | 500.6 g/mol |
|---|---|
| Molecular Formula | C25H31F3O5S |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 500.18442974 g/mol |
| Monoisotopic Mass | 500.18442974 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 984 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 14 | |
|---|---|
| Drug Name | Cutivate |
| PubMed Health | Fluticasone |
| Drug Classes | Anti-Inflammatory, Corticosteroid, Intermediate, Corticosteroid, Strong |
| Drug Label | CUTIVATE (fluticasone propionate cream) Cream, 0.05% contains fluticasone propionate [(6,11,16,17)-6,9,-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid, S-fluoromethyl ester], a synthetic fluorina... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Lotion; Ointment |
| Route | Topical |
| Strength | 0.05%; 0.005% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 2 of 14 | |
|---|---|
| Drug Name | Flonase |
| Drug Label | The active component of FLOVENT HFA 44 mcg Inhalation Aerosol, FLOVENT HFA 110 mcg Inhalation Aerosol, and FLOVENT HFA 220 mcg Inhalation Aerosol is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6,9-difluoro-11... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.05mg/spray |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 14 | |
|---|---|
| Drug Name | Flovent diskus 100 |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.1mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 4 of 14 | |
|---|---|
| Drug Name | Flovent diskus 250 |
| Drug Label | Fluticasone propionate, the active component of Fluticasone Propionate Nasal Spray USP, is a synthetic corticosteroid having the chemical name S-(fluoromethyl)6,9-difluoro-11-17-dihydroxy-16-methyl-3-oxoandrosta-1,4-diene-17-carbothioate, 17-... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.25mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 5 of 14 | |
|---|---|
| Drug Name | Flovent diskus 50 |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.05mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 6 of 14 | |
|---|---|
| Drug Name | Flovent hfa |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Aerosol, metered |
| Route | Inhalation |
| Strength | 0.22mg/inh; 0.11mg/inh; 0.044mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 7 of 14 | |
|---|---|
| Drug Name | Fluticasone propionate |
| Drug Label | Fluticasone propionate, the active component of FLONASE Nasal Spray, is a synthetic corticosteroid having the chemical name S-(fluoromethyl)6,9-difluoro-11-17-dihydroxy-16-methyl-3-oxoandrosta-1,4-diene-17-carbothioate, 17-propionate and the... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Ointment; Spray, metered; Cream; Lotion |
| Route | Nasal; Topical |
| Strength | 0.05%; 0.005%; 0.05mg/spray |
| Market Status | Prescription |
| Company | Wockhardt; Apotex; Roxane; Glenmark Generics; Fougera Pharms; Hi Tech Pharma; Perrigo New York; Tolmar; G And W Labs; Perrigo Israel |
| 8 of 14 | |
|---|---|
| Drug Name | Flovent diskus 250 |
| Drug Label | Fluticasone propionate, the active component of Fluticasone Propionate Nasal Spray USP, is a synthetic corticosteroid having the chemical name S-(fluoromethyl)6,9-difluoro-11-17-dihydroxy-16-methyl-3-oxoandrosta-1,4-diene-17-carbothioate, 17-... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.25mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 9 of 14 | |
|---|---|
| Drug Name | Flovent diskus 50 |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.05mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 10 of 14 | |
|---|---|
| Drug Name | Flovent hfa |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Aerosol, metered |
| Route | Inhalation |
| Strength | 0.22mg/inh; 0.11mg/inh; 0.044mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 11 of 14 | |
|---|---|
| Drug Name | Fluticasone propionate |
| Drug Label | Fluticasone propionate, the active component of FLONASE Nasal Spray, is a synthetic corticosteroid having the chemical name S-(fluoromethyl)6,9-difluoro-11-17-dihydroxy-16-methyl-3-oxoandrosta-1,4-diene-17-carbothioate, 17-propionate and the... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Ointment; Spray, metered; Cream; Lotion |
| Route | Nasal; Topical |
| Strength | 0.05%; 0.005%; 0.05mg/spray |
| Market Status | Prescription |
| Company | Wockhardt; Apotex; Roxane; Glenmark Generics; Fougera Pharms; Hi Tech Pharma; Perrigo New York; Tolmar; G And W Labs; Perrigo Israel |
| 12 of 14 | |
|---|---|
| Drug Name | Cutivate |
| PubMed Health | Fluticasone |
| Drug Classes | Anti-Inflammatory, Corticosteroid, Intermediate, Corticosteroid, Strong |
| Drug Label | CUTIVATE (fluticasone propionate cream) Cream, 0.05% contains fluticasone propionate [(6,11,16,17)-6,9,-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid, S-fluoromethyl ester], a synthetic fluorina... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Lotion; Ointment |
| Route | Topical |
| Strength | 0.05%; 0.005% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 13 of 14 | |
|---|---|
| Drug Name | Flonase |
| Drug Label | The active component of FLOVENT HFA 44 mcg Inhalation Aerosol, FLOVENT HFA 110 mcg Inhalation Aerosol, and FLOVENT HFA 220 mcg Inhalation Aerosol is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6,9-difluoro-11... |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.05mg/spray |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 14 of 14 | |
|---|---|
| Drug Name | Flovent diskus 100 |
| Active Ingredient | Fluticasone propionate |
| Dosage Form | Powder |
| Route | Inhalation |
| Strength | 0.1mg/inh |
| Market Status | Prescription |
| Company | Glaxo Grp |
Fluticasone propionate is indicated as an inhaler for the treatment and management of asthma by prophylaxisas well as inflammatory and pruritic dermatoses. Fluticasone propionate nasal spray is indicated for managing allergic and nonallergic rhinitis.
FDA Label
Treatment of asthma
Systemically, fluticasone propionate activates glucocorticoid receptors, and inhibits lung eosinophilia in rats. Fluticasone propionate as a topical formulation is also associated with vasoconstriction in the skin.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Absorption
Intranasal bioavailability of fluticasone propionate is <2%, and oral bioavailability is <1%. Intranasal exposure results in the majority of the dose being swallowed. Topical absorption of fluticasone propionate is very low but can change depending on a number of factors including integrity of the skin and the presence of inflammation or disease. A study of 24 healthy Caucasian males showed an inhaled bioavailability of 9.0%.
Route of Elimination
Fluticasone propionate is mainly eliminated in the feces with <5% eliminated in the urine.
Volume of Distribution
The volume of distribution of intravenous fluticasone propionate is 4.2L/kg. A study of 24 healthy Caucasian males showed a volume of distribution at steady state of 577L following intravenous administration.
Clearance
1093mL/min for fluticasone propionate. A study of 24 healthy Caucasian males showed a clearance of 63.9L/h following intravenous administration.
Fluticasone propionate is cleared from hepatic metabolism by cytochrome P450 3A4. Fluticasone propionate is hydrolysed at the FIVE-S-fluoromethyl carbothioate group, forming an inactive metabolite.
7.8 hours for intravenous fluticasone propionate. A study of 24 healthy Caucasian males shows a half life of 14.0 hours following intravenous administration and 10.8 hours following inhalation.
Fluticasone propionate works through an unknown mechanism to affect the action of various cell types and mediators of inflammation. Fluticasone propionate activates glucocorticoid receptors and inhibits lung eosinophilia in rats.