


1. Nuc-3073
1. Nuc-3373
2. 1332837-31-6
3. 4yo6qt3sz9
4. Cpf-373
5. Nuc-3073
6. Fosifloxuridine Nafalbenamide [usan]
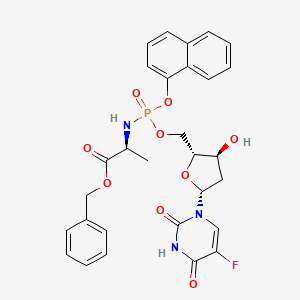
7. Benzyl (2s)-2-[[[(2r,3s,5r)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy-naphthalen-1-yloxyphosphoryl]amino]propanoate
8. Unii-4yo6qt3sz9
9. Chembl2181367
10. Ex-a4413
11. Who 10749
12. Db14859
13. Fosifloxuridine Nafalbenamide [inn]
14. Hy-109115
15. Cs-0078080
16. Fosifloxuridine Nafalbenamide [who-dd]
17. J3.616.613d
18. A936616
19. Benzyl N-[p-ambo-2'-deoxy-5-fluoro-p-(naphthalen-1-yl)-5'-uridyly]}-l-alaninate
20. L-alanine, N-(2'-deoxy-5-fluoro-p-1-naphthalenyl-5'-uridylyl)-, Phenylmethyl Ester
21. N-(2'-deoxy-5-fluoro-p-1-naphthalenyl-5'-uridylyl)-l-alanine Phenylmethyl Ester
22. Benzyl (2s)-2-{[{[(2r,3s,5r)-5-(5-fluoro-2,4-dioxo-3,4-dihydro-1(2h)-pyrimidinyl)-3-hydroxytetrahydro-2-furanyl]methoxy}(1-naphthyloxy)phosphoryl]amino}propanoate
23. L-alanine, N-(-2'-deoxy-2',2'-difluoro-p-1-naphthalenyl-5'-cytidylyl)-, Phenylmethyl Ester
| Molecular Weight | 613.5 g/mol |
|---|---|
| Molecular Formula | C29H29FN3O9P |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Exact Mass | 613.16254467 g/mol |
| Monoisotopic Mass | 613.16254467 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |