

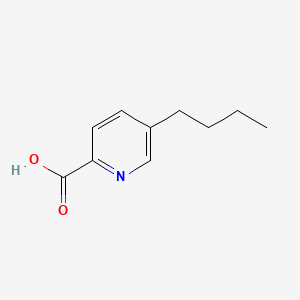

1. 5 Butyl 2 Pyridinedicarboxylic Acid
2. 5-butyl-2-pyridinedicarboxylic Acid
3. Acid, 5-butyl-2-pyridinedicarboxylic
4. Acid, Fusaric
5. Calcium Fusarate
6. Fusarate, Calcium
1. 5-butylpicolinic Acid
2. 536-69-6
3. 5-butylpyridine-2-carboxylic Acid
4. Fusarinic Acid
5. 2-pyridinecarboxylic Acid, 5-butyl-
6. 5-butyl-2-pyridinecarboxylic Acid
7. Picolinic Acid, 5-butyl-
8. 5-n-butylpyridine-2-carboxylic Acid
9. 5-butyl-pyridine-2-carboxylic Acid
10. Chembl24510
11. Chebi:5199
12. Jwj963070n
13. Nsc19870
14. Tnp00268
15. Nsc-19870
16. Ncgc00015441-04
17. Cas-536-69-6
18. 5-butylpyridine-3-carboxylic Acid
19. Hsdb 3487
20. Einecs 208-643-0
21. Mfcd00006298
22. Nsc 19870
23. Brn 0125804
24. Unii-jwj963070n
25. Prestwick_233
26. 5-n-butylpicolinic Acid
27. Prestwick0_000442
28. Prestwick1_000442
29. Prestwick2_000442
30. Prestwick3_000442
31. 5-n-butyl Picolinic Acid
32. Lopac-f-6513
33. Dsstox_cid_3085
34. Fusaric Acid [mi]
35. Fusaric Acid [jan]
36. Dsstox_rid_76868
37. Fusaric Acid [hsdb]
38. Dsstox_gsid_23085
39. Lopac0_000526
40. Oprea1_115508
41. Wln: T6nj Bvq E4
42. 5-n-butyl-2-picolinic Acid
43. Bspbio_000484
44. 5-22-02-00384 (beilstein Handbook Reference)
45. Mls002153813
46. Fusaric Acid [mart.]
47. Schembl178006
48. Spbio_002423
49. Bpbio1_000534
50. Dtxsid5023085
51. Hms1569i06
52. Hms2096i06
53. Hms2230m05
54. Hms3261j13
55. Hms3369p03
56. Zinc1531682
57. Tox21_110149
58. Tox21_500526
59. Bdbm50000439
60. Stl564384
61. Akos015891748
62. Ccg-204616
63. Fusaric Acid, From Gibberella Fujikuroi
64. Lp00526
65. Sdccgsbi-0050509.p002
66. Ncgc00015441-01
67. Ncgc00015441-02
68. Ncgc00015441-03
69. Ncgc00015441-05
70. Ncgc00015441-06
71. Ncgc00015441-07
72. Ncgc00015441-08
73. Ncgc00015441-10
74. Ncgc00015441-15
75. Ncgc00093919-01
76. Ncgc00093919-02
77. Ncgc00093919-03
78. Ncgc00261211-01
79. As-57621
80. Smr001233184
81. Db-052375
82. Hy-128483
83. Cs-0099145
84. Eu-0100526
85. F0227
86. Ft-0626585
87. A19903
88. F 6513
89. F-9000
90. T72585
91. 5-butyl-pyridine-2-carboxylic Acid (fusaric Acid)
92. Q905703
93. Sr-01000075634
94. Sr-01000075634-1
95. Brd-k87049188-001-03-6
96. Fusaric Acid, For Hplc Derivatization, >=99.0% (hplc)
97. 4-(acetylamino)-6-nitro-1,3-benzenedicarboxylicacid
98. Cqv
| Molecular Weight | 179.22 g/mol |
|---|---|
| Molecular Formula | C10H13NO2 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 179.094628657 g/mol |
| Monoisotopic Mass | 179.094628657 g/mol |
| Topological Polar Surface Area | 50.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dopamine Agents; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
EXPTL USE: FUSARIC ACID (100 MG/KG, IP) GIVEN 1.5 HR PRIOR TO WATER-IMMERSION STRESS ALMOST COMPLETELY PREVENTED GASTRIC ULCER FORMATION IN RATS. FUSARIC ACID PROBABLY PREVENTS GASTRIC ULCERATION BY DECREASING NORADRENALINE RELEASE IN THE CNS.
OSUMI Y ET AL; PREVENTIVE EFFECT OF FUSARIC ACID, A DOPAMINE BETA-HYDROXYLASE INHIBITOR, ON THE GASTRIC ULCERATION INDUCED BY WATER-IMMERSION STRESS IN RATS; JAP J PHARMACOL 23(6) 904 (1973)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Dopamine Agents
Any drugs that are used for their effects on dopamine receptors, on the life cycle of dopamine, or on the survival of dopaminergic neurons. (See all compounds classified as Dopamine Agents.)
THE METABOLISM OF (14)C-LABELED FUSARIC ACID WAS STUDIED IN MALE & PREGNANT RATS AFTER ORAL ADMIN OF 20 MG/KG. THE MAJOR PART OF RADIOACTIVITY RETAINED IN THE BODY OF MALE RATS WAS IN THE KIDNEY, LIVER, & PLASMA 30 MIN AFTER ADMIN, & DECLINED RAPIDLY THEREAFTER. MOST (92.9%) OF THE DOSE APPEARED IN URINE BY 24 HR AFTER ADMIN & 93.1% BY 48 HR. A CONSIDERABLE AMOUNT OF RADIOACTIVITY APPEARED IN BILE WITHIN 1 HR AFTER ADMIN. AN EASY TRANSFER OF RADIOACTIVITY INTO THE FETUS WAS SHOWN BY RADIOAUTOGRAPHY OF PREGNANT RATS. THE ACTIVITY WAS NOT DETECTED IN THE FETUS IN 24 HR.
MATSUZAKI M ET AL; ABSORPTION, DISTRIBUTION AND EXCRETION OF 14C-FUSARIC ACID IN THE RAT; JPN J ANTIBIOT 29(5) 456 (1976)
ZINC, COBALT, & MOLYBDENUM ENHANCE THE BIOSYNTHESIS OF FUSARIC ACID BY FUSARIUM OXYSPORUM. NICOTINIC ACID WAS SLIGHTLY STIMULATORY, & TRYPTOPHAN, CYSTEINE, & THE COMBINATION OF INDOLEACETATE & SERINE MARKEDLY STIMULATED THE SYNTHESIS.
YASAKOVA EI, BEKKER ZE; EFFECT OF NITROGEN-CONTAINING COMPOUNDS AND TRACE ELEMENTS ON BIOSYNTHESIS OF FUSARIC ACID IN LABORATORY CULTURE; EKOL FIZIOL METODY BORBE FUZARIOZNYM VILTOM KHLOP 2: 190 (1973)
INDOLEACETIC ACID ALONE INHIBITED FUSARIC ACID FORMATION BY FUSARIUM OXYSPORUM, BUT INDOLEACETATE WITH SERINE HAD A STIMULATORY EFFECT. THE COMBINATION OF INDOLEACETATE & SERINE WITH TRYPTOPHAN INHIBITED THE BIOSYNTHESIS. HOMOSERINE SHOWED STIMULATORY ACTIVITY INDEPENDENT OF THE OTHER COMPOUNDS TESTED SINCE IT WAS NOT AFFECTED BY THEIR PRESENCE.
AZIMOVA RM; EFFECT OF AMINO ACIDS PARTICIPATING IN BIOGENESIS OF PYRIDINES ON BIOSYNTHESIS OF FUSARIC ACID; EKOL FIZIOL METODY BORBE FUZARIOZNYM VILTOM KHLOP 2: 200 (1973)
THE BIOSYNTHETIC PATHWAY FOR FUSARIC ACID WAS INVESTIGATED USING 1-(13)C-LABELED & 2-(13)C-LABELED ASPARTATE. CARBON ATOMS 2, 3, 4, & 7 WERE DERIVED FROM ACETATE VIA ASPARTATE OR A RELATED C4 DICARBOXYLIC ACID, WHEREAS CARBONS 5, 6, 8, 9, 10, & 11 WERE DERIVED MORE DIRECTLY FROM ACETATE. ASPARTIC ACID APPARENTLY IS METABOLIZED TO FUSARIC ACID VIA OXALOACETATE, & L-ASPARTATE SERVES AS A DONOR OF NITROGEN, IN AN AMINOTRANSFERASE REACTION, TO A SEPARATE OXALACETATE POOL OF PRIMARILY ENDOGENOUS ORIGIN.
DESATY D ET AL; USE OF CARBON-13 IN BIOSYNTHETIC STUDIES. INCORPORATION OF ISOTOPICALLY LABELED ACETATE AND ASPARTATE INTO FUSARIC ACID; CAN J BIOCHEM 46(10) 1293 (1968)
IN RATS, THE MAJOR METABOLITE OF 5-(N-BUTYL)PICOLINAMIDE IS FUSARIC ACID, WHICH IS A DOPAMINE-BETA-HYDROXYLASE INHIBITOR. HENCE, ADMIN OF THE DRUG LOWERS THE CONCN OF ENDOGENOUS L-NORADRENALINE IN THE BRAIN, HEART, & SPLEEN.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 92
FUSARIC ACID SUPPRESSED RAPID EYE MOVEMENT (REM) SLEEP IN CATS BUT HAD NO SIGNIFICANT EFFECT ON SLOW WAVE SLEEP. REM SLEEP USUALLY REBOUNDED AFTER A PERIOD OF DRUG-INDUCED SUPPRESSION, INDICATING THAT, ALTHOUGH FUSARIC ACID SUPPRESSED THE PERIPHERAL MANIFESTATIONS OF REM, THE BIOLOGICAL NEED FOR REM WAS NOT ALTERED.
SATOH T, TANAKA R; SELECTIVE SUPPRESSION OF RAPID EYE MOVEMENT SLEEP (REM) BY FUSARIC ACID, AN INHIBITOR OF DOPAMINE BETA-OXIDASE; EXPERIENTIA 29(2) 177 (1973)
FUSARIC ACID INHIBITED NORADRENALINE & DOPAMINE UPTAKE IN SYNAPTOSOMES FROM RAT HYPOTHALAMUS & CORPUS STRIATUM. THE BASAL OVERFLOW OF NORADRENALINE & DOPAMINE FROM BRAIN STEM & CORPUS STRIATUM SLICES WAS STIMULATED BY FUSARIC ACID. THE DATA SHOW THAT FUSARIC ACID, A DOPAMINE-BETA-HYDROXYLASE INHIBITOR, ALSO EXERTS MARKED EFFECTS IN THE CNS BY INTERFERING WITH OTHER SYNAPTOSOMAL FUNCTIONS.
FISCHER HD ET AL; INTERACTION AT THE SYNAPTIC LEVEL OF FUSARIC ACID WITH NEUROTRANSMITTERS; ACTA BIOL MED GER 39(8-9) 935 (1980)
FUSARIC ACID (100 MG/KG, IP) INCREASED THE LEVELS OF TRYPTOPHAN, SEROTONIN, & 5-HYDROXYINDOLEACETIC ACID IN RAT BRAIN & THE LEVEL OF FREE TRYPTOPHAN IN THE BLOOD INDICATING THAT IN ADDITION TO ITS CNS EFFECT, FUSARIC ACID EXERTS A PERIPHERAL ACTION ON SEROTONIN METABOLISM BY INHIBITING TRYPTOPHAN BINDING TO SERUM ALBUMIN.
BONNAY MM ET AL; PERIPHERAL EFFECT OF FUSARIC ACID, A DOPAMINE BETA-HYDROXYLASE INHIBITOR, ON SEROTONIN METABOLISM; BIOCHEM PHARMACOL 23(19) 2770 (1974)
FUSARIC ACID (75 MG/KG, IP), AN INHIBITOR OF DOPAMINE BETA-HYDROXYLASE, EFFECTIVE IN THE RELIEF OF TREMORS, RIGIDITY, & SPEECH DIFFICULTIES ASSOCIATED WITH PARKINSONS DISEASE, INCREASED THE BRAIN SEROTONIN LEVELS & DECREASED THE BRAIN NORADRENALINE LEVELS OF RATS.
HIDAKA H; FUSARIC (5-BUTYLPICOLINIC) ACID, AN INHIBITOR OF DOPAMINE BETA-HYDROXYLASE, AFFECTS SEROTONIN AND NORADRENALINE; NATURE (LONDON) 231(5297) 54 (1971)
FUSARIC ACID (FA) INCREASED MONOSYNAPTIC REFLEX NEURAL ACTIVITY IN A DOSE-DEPENDENT MANNER IN CATS. FA DID NOT INCREASE THE BLOOD PRESSURE BUT INHIBITED THE SYNTHESIS OF NOREPINEPHRINE FROM DOPAMINE.
WATANABE Y ET AL; EFFECTS OF FUSARIC (5-BUTYLPICOLINIC) ACID ON THE MONOSYNAPTIC REFLEX NEURAL ACTIVITY OF CAT SPINAL CORD; TOKAI J EXP CLIN MED 6(4) 443 (1981)