

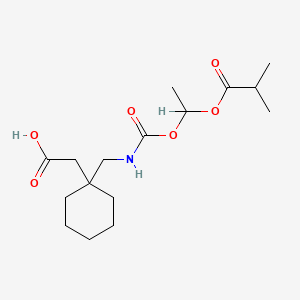

1. 1-(((alpha-isobutanoyloxyethoxy)carbonyl)aminomethyl)-1-cyclohexaneacetic Acid
2. Xp 10569
3. Xp 11084
4. Xp 11239
5. Xp 12464
6. Xp 13512
7. Xp-10569
8. Xp-11084
9. Xp-11239
10. Xp-12464
11. Xp-13512
12. Xp10569
13. Xp11084
14. Xp11239
15. Xp12464
16. Xp13512
1. 478296-72-9
2. Horizant
3. Regnite
4. Xp-13512
5. Solzira
6. Xp13512
7. Asp8825
8. Xp 13512
9. Gsk1838262
10. Asp-8825
11. 75ocl1spbq
12. Gsk-1838262
13. Chebi:68840
14. 2-(1-((((1-(isobutyryloxy)ethoxy)carbonyl)amino)methyl)cyclohexyl)acetic Acid
15. 2-[1-[[1-(2-methylpropanoyloxy)ethoxycarbonylamino]methyl]cyclohexyl]acetic Acid
16. {1-[({[1-(isobutyryloxy)ethoxy]carbonyl}amino)methyl]cyclohexyl}acetic Acid
17. (1-(((((1rs)-1-((2-methylpropanoyl)oxy)ethoxy)carbonyl)amino)methyl)cyclohexyl)acetic Acid
18. (1-{[({(1rs)-1-[isobutyryloxy]ethoxy}carbonyl) Amino]methyl}cyclohexyl)acetic Acid
19. Cyclohexaneacetic Acid, 1-((((1-(2-methyl-1-oxopropoxy)ethoxy)carbonyl)amino)methyl)-
20. Gabapentin Encarbil
21. Unii-75ocl1spbq
22. Gsk 1838262
23. Gabapentin Enacarbil [usan:inn]
24. Gabapentin-encarbil
25. Horizant (tn)
26. Asp 8825
27. Gabapentine Enacarbil
28. Gabapentina Enacarbilo
29. Gabapentinum Enacarbilum
30. Schembl25455
31. Gtpl7560
32. Chembl1628502
33. Gabapentin Encarbil [mi]
34. Dtxsid30870359
35. Gabapentin Enacarbil [inn]
36. Gabapentin Enacarbil [jan]
37. Gabapentin Enacarbil [usan]
38. Bcp12845
39. Gabapentin Enacarbil [vandf]
40. Gabapentin Enacarbil [mart.]
41. Mfcd09954124
42. Xp-053
43. Gabapentin Enacarbil [who-dd]
44. Akos025290727
45. Gabapentin Enacarbil (jan/usan/inn)
46. Cs-1698
47. Db08872
48. Pb32822
49. 1-[[[[1-(2-methyl-1-oxopropoxy)ethoxy]carbonyl]amino]methyl]cyclohexaneacetic Acid
50. Ncgc00532500-01
51. Ac-27644
52. As-35192
53. Gabapentin Enacarbil [orange Book]
54. Hy-16216
55. Db-070861
56. Ft-0753836
57. A20372
58. D09539
59. 296g729
60. Q5515257
61. {[(1-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane Acetic Acid
62. {[(alpha-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane Acetic Acid
63. 1-{[(alpha-isobutanovloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane Acetic Acid
64. 1-{[(alpha-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane Acetic Acid
65. 1-{[(alpha-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexaneacetic Acid
66. 2-[1-[[[1-[(2-methylpropanoyl)oxy]ethoxy]carbonylamino]methyl]cyclohexyl]acetic Acid
| Molecular Weight | 329.39 g/mol |
|---|---|
| Molecular Formula | C16H27NO6 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 329.18383758 g/mol |
| Monoisotopic Mass | 329.18383758 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Horizant |
| Drug Label | HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin. Gabapentin enacarbil is described as (1-{[({(1RS)-1-[(2-Methylpropanoyl)oxy]ethoxy}carbonyl)amino]methyl} cyclohexyl) acetic acid. It has a molecular formula of C16H27NO6 and a molecular wei... |
| Active Ingredient | Gabapentin enacarbil |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg; 300mg |
| Market Status | Prescription |
| Company | Xenoport |
| 2 of 2 | |
|---|---|
| Drug Name | Horizant |
| Drug Label | HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin. Gabapentin enacarbil is described as (1-{[({(1RS)-1-[(2-Methylpropanoyl)oxy]ethoxy}carbonyl)amino]methyl} cyclohexyl) acetic acid. It has a molecular formula of C16H27NO6 and a molecular wei... |
| Active Ingredient | Gabapentin enacarbil |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg; 300mg |
| Market Status | Prescription |
| Company | Xenoport |
For the treatment of adult Restless Legs Syndrome (RLS) and postherpetic neuralgia (PHN).
FDA Label
Since gabapentin enacarbil is a prodrug of gabapentin, it's physiological effects are the same as gabapentin. Concerning PHN, gabapentin prevents allodynia and hyperalgesia.
Absorption
Gabapentin enacarbil is absorbed in the intestines by active transport through the proton-linked monocarboxylate transporter, MCT-1.
Route of Elimination
Gabapentin enacarbil is eliminated primarily in the urine (94%) and to a lesser extent in the feces (5%).
Volume of Distribution
The volume of distribution is 76L.
Clearance
Renal clearance of gabapentin is 5 to 7 L/hr.
Gabapentin enacarbil does not interact with any of the major cytochrome P450 enzymes.
The elimination half-life of gabapentin is 5.1 to 6.0 hours.
Although the exact mechanism of action of gabapentin in RLS and PHN is unknown, it is presumed to involve the descending noradrenergic system, resulting in the activation of spinal alpha2-adrenergic receptors.