
1. Gadofosveset
2. Gadofosveset Trisodium
3. Ms 325
4. Ms-325
5. Vasovist
1. Gadofosveset Trisodium
2. 193901-90-5
3. Gadofosveset Trisodium (usan)
4. 211570-55-7
5. Ablavar (tn)
6. Dtxsid501027793
7. D04286
8. Q5516411
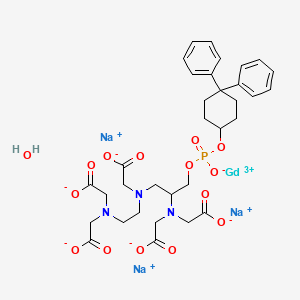
9. Gadolinium Sodium 4-(bis(carboxylatomethyl)amino)-6,9-bis(carboxylatomethyl)-1-((4,4-diphenylcyclohexyl)oxy)-1-oxido-2-oxa-6,9-diaza-1-phosphaundecan-11-oate 1-oxide Hydrate (1:3:1:1)
| Molecular Weight | 975.9 g/mol |
|---|---|
| Molecular Formula | C33H40GdN3Na3O15P |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 18 |
| Exact Mass | 976.11312 g/mol |
| Monoisotopic Mass | 976.11312 g/mol |
| Topological Polar Surface Area | 270 Ų |
| Heavy Atom Count | 56 |
| Formal Charge | 0 |
| Complexity | 1130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 6 |
| 1 of 2 | |
|---|---|
| Drug Name | Ablavar |
| PubMed Health | Gadofosveset (Injection) |
| Drug Classes | Diagnostic Agent, Radiological Contrast Media |
| Drug Label | ABLAVAR (gadofosveset trisodium) Injection is a sterile, nonpyrogenic, formulation of a stable gadolinium diethylenetriaminepentaacetic acid (GdDTPA) chelate derivative with a diphenylcyclohexylphosphate group. Each mL of ABLAVAR Injection contains 2... |
| Active Ingredient | Gadofosveset trisodium |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 3660mg/15ml (244mg/ml); 2440mg/10ml (244mg/ml) |
| Market Status | Prescription |
| Company | Lantheus Medcl |
| 2 of 2 | |
|---|---|
| Drug Name | Ablavar |
| PubMed Health | Gadofosveset (Injection) |
| Drug Classes | Diagnostic Agent, Radiological Contrast Media |
| Drug Label | ABLAVAR (gadofosveset trisodium) Injection is a sterile, nonpyrogenic, formulation of a stable gadolinium diethylenetriaminepentaacetic acid (GdDTPA) chelate derivative with a diphenylcyclohexylphosphate group. Each mL of ABLAVAR Injection contains 2... |
| Active Ingredient | Gadofosveset trisodium |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 3660mg/15ml (244mg/ml); 2440mg/10ml (244mg/ml) |
| Market Status | Prescription |
| Company | Lantheus Medcl |
Ablavar is indicated for use as a contrast agent in magnetic resonance angiography (MRA) to evaluate aortoiliac occlusive disease (AIOD) in adults with known or suspected peripheral vascular disease.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
/BOXED WARNING/ WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF) Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. The risk for NSF appears highest among patients with: chronic, severe kidney disease (GFR < 30 mL/min/1.73m sq), or acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. For patients at highest risk for NSF, do not exceed the recommended ABLAVAR dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
In patients with renal insufficiency, acute renal failure requiring dialysis or worsening renal function have occurred with the use of other gadolinium agents. The risk of renal failure may increase with increasing dose of gadolinium contrast. Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. Consider follow-up renal function assessments for patients with a history of renal dysfunction. No reports of acute renal failure were observed in clinical trials of ABLAVAR.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
In clinical trials, a small increase (2.8 msec) in the average change from baseline in QTc was observed at 45 minutes following Ablavar administration; no increase was observed at 24 and 72 hours. A QTc change of 30 to 60 msec from baseline was observed in 39/702 (6%) patients at 45 min following Ablavar administration. At this time point, 3/702 (0.4%) patients experienced a QTc increase of > 60 msec. These QTc prolongations were not associated with arrhythmias or symptoms. In patients at high risk for arrhythmias due to QTc prolongation (e.g., concomitant medications, underlying cardiac conditions) consider obtaining baseline electrocardiograms to help assess the risks for Ablavar administration. If Ablavar is administered to these patients, consider follow-up electrocardiograms and risk reduction measures (e.g., patient counseling or intensive electrocardiography monitoring) until most Ablavar has been eliminated from the blood. In patients with normal renal function, most Ablavar was eliminated from the blood by 72 hours following injection.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
Ablavar may cause anaphylactoid and/or anaphylactic reactions, including life-threatening or fatal reactions. In clinical trials, anaphylactoid and/or anaphylactic reactions occurred in two of 1676 subjects. If anaphylactic or anaphylactoid reactions occur, stop ABLAVAR Injection and immediately begin appropriate therapy. Observe patients closely, particularly those with a history of drug reactions, asthma, allergy or other hypersensitivity disorders, during and up to several hours after ablavar administration. Have trained personnel and emergency resuscitative equipment available prior to and during ablavar administration. If such a reaction occurs stop ablavar and immediately begin appropriate therapy.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
For more Drug Warnings (Complete) data for Gadofosveset trisodium (9 total), please visit the HSDB record page.
This medicinal product is for diagnostic use only.
Ablavar is indicated for contrast-enhanced magnetic resonance angiography (CE-MRA) for visualisation of abdominal or limb vessels in adults only, with suspected or known vascular disease.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08CA
Gadofosveset is eliminated primarily in the urine, with between 79% and 94% (mean of 83.7%) of an injected dose recovered in the urine. Of the total gadofosveset recovered in urine, 94% is recovered within the first 72 hours. A small portion of gadofosveset dose is recovered in feces (approximately 4.7%).
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
The mean volume of distribution at steady state for gadofosveset was 148 +/- 16 mL/kg, roughly equivalent to that of extracellular fluid. A significant portion of circulating gadofosveset is bound to plasma proteins, predominantly albumin. At 0.05, 0.5, 1 and 4 hours after injection of 0.03 mmol/kg the plasma protein binding of gadofosveset ranges from 79.8 to 87.4%.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
The pharmacokinetics of intravenously administered gadofosveset conforms to a two-compartment open model with mean plasma concentrations (reported as mean +/-SD) of 0.43 +/- 0.04 mmol/L at 3 minutes post-injection, and 0.24 +/- 0.03 mmol/L at one hour post-injection. The mean half-life of the distribution phase is 0.48 +/- 0.11 hours and the mean half-life of the elimination phase is 16.3 +/- 2.6 hours. The mean total clearance of gadofosveset is 6.57 +/- 0.97 mL/h/kg following the administration of 0.03 mmol/kg.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
In animal studies, less than 1% of gadofosveset at doses up to 0.3 mmol/kg was secreted in the milk of lactating rats.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
Gadofosveset does not undergo measurable metabolism in humans.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d
The mean half-life of the distribution phase is 0.48 +/- 0.11 hours and the mean half-life of the elimination phase is 16.3 +/- 2.6 hours.
NIH; DailyMed. Current Medication Information for Ablavar-(gadofosveset trisodium injection, solution) (Updated: August 2013). Available from, as of March 13, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8175d0e7-b627-6c68-73e6-d1fc7660775d