
1. Gadoversetamide
1. Gadoversetamide
2. Gadoversetamide (200 Mg)
3. 131069-91-5
4. Rlm74t3z9d
5. Mp-1177
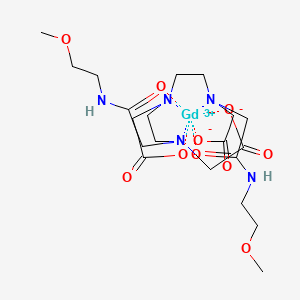
6. (n,n-bis(2-((carboxymethyl)(((2-methoxyethyl)carbamoyl)methyl)amino)ethyl)glycinato(3-))gadolinium
7. Unii-rlm74t3z9d
8. Optimark In Plastic Container
9. Hsdb 7550
10. Gadoversetamide [usan:usp:inn:ban]
11. (8,11-bis(carboxymethyl)-14-(2-((2-methoxyethyl)amino)-2-oxoethyl)-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oato(3-)), Gadolinium
12. Dtxsid10156865
13. (gadoversetamide) (8,11-bis(carboxymethyl)-14-(2-((2-methoxyethyl)amino)-2-oxoethyl)-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oato(3-)), Gadolinium
| Molecular Weight | 661.8 g/mol |
|---|---|
| Molecular Formula | C20H34GdN5O10 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 6 |
| Exact Mass | 662.15468 g/mol |
| Monoisotopic Mass | 662.15468 g/mol |
| Topological Polar Surface Area | 207 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 637 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Optimark |
| Active Ingredient | Gadoversetamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1654.5mg/5ml (330.9mg/ml); 6618mg/20ml (330.9mg/ml); 4963.5mg/15ml (330.9mg/ml); 3309mg/10ml (330.9mg/ml); 16.545gm/50ml (330.9mg/ml) |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 2 of 4 | |
|---|---|
| Drug Name | Optimark in plastic container |
| PubMed Health | Gadoversetamide (Injection) |
| Drug Classes | Diagnostic Agent, Radiological Contrast Media, Radiological Ionic Contrast Media, Radiological Non-Ionic Contrast Media |
| Drug Label | Optimark (gadoversetamide) injection is a nonionic gadolinium chelate of diethylenetriamine pentaacetic acid bismethoxyethylamide (gadoversetamide), for intravenous injection.Optimark injection is provided as a sterile, preservative-free, nonpyrogeni... |
| Active Ingredient | Gadoversetamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6618mg/20ml (330.9mg/ml); 4963.5mg/15ml (330.9mg/ml); 3309mg/10ml (330.9mg/ml); 9927mg/30ml (330.9mg/ml) |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 3 of 4 | |
|---|---|
| Drug Name | Optimark |
| Active Ingredient | Gadoversetamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1654.5mg/5ml (330.9mg/ml); 6618mg/20ml (330.9mg/ml); 4963.5mg/15ml (330.9mg/ml); 3309mg/10ml (330.9mg/ml); 16.545gm/50ml (330.9mg/ml) |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 4 of 4 | |
|---|---|
| Drug Name | Optimark in plastic container |
| PubMed Health | Gadoversetamide (Injection) |
| Drug Classes | Diagnostic Agent, Radiological Contrast Media, Radiological Ionic Contrast Media, Radiological Non-Ionic Contrast Media |
| Drug Label | Optimark (gadoversetamide) injection is a nonionic gadolinium chelate of diethylenetriamine pentaacetic acid bismethoxyethylamide (gadoversetamide), for intravenous injection.Optimark injection is provided as a sterile, preservative-free, nonpyrogeni... |
| Active Ingredient | Gadoversetamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6618mg/20ml (330.9mg/ml); 4963.5mg/15ml (330.9mg/ml); 3309mg/10ml (330.9mg/ml); 9927mg/30ml (330.9mg/ml) |
| Market Status | Prescription |
| Company | Mallinckrodt |
Contrast Media
National Library of Medicine's Medical Subject Headings. Gadoversetamide. Online file (MeSH, 2014). Available from, as of October 23, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Optimark is indicated for use with magnetic resonance imaging (MRI) in patients with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
Optimark is indicated for use with MRI to provide contrast enhancement and facilitate visualization of lesions with abnormal vascularity in the liver of patients who are highly suspect for liver structural abnormalities on computed tomography. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
/BOXED WARNING/ WARNING: NEPHROGENIC SYSTEMIC FIBROSIS. Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Do not administer Optimark to patients with: chronic, severe kidney disease (GFR <30 mL/min/1.73 sq m), or acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. Do not exceed the recommended Optimark dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73sq m) as well as patients with acute kidney injury. Do not administer Optimark to these patients. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 to 59 mL/min/1.73 sq m) and little, if any, for patients with chronic, mild kidney disease (GFR 60 to 89 mL/min/1.73 sq m). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. ... Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury, or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronic kidney disease (e.g., age >60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing. Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. When administering Optimark, do not exceed the recommended dose and allow a sufficient period of time for elimination of the drug prior to any re-administration.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
Severe hypersensitivity reactions including anaphylaxis have been observed with administration of gadolinium products including Optimark. Before administering Optimark ensure the availability of resuscitation equipment and personnel trained in resuscitation techniques. Patients with a history of allergy, drug reactions or other hypersensitivity-like disorders may be at greater risk and should be closely observed during the procedure and for several hours after drug administration. If a reaction occurs, stop Optimark and immediately begin appropriate therapy including resuscitation.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
For more Drug Warnings (Complete) data for GADOVERSETAMIDE (14 total), please visit the HSDB record page.
This medicinal product is for diagnostic use only.
Optimark is indicated for use with magnetic resonance imaging (MRI) of the central nervous system (CNS) and liver. It provides contrast enhancement and facilitates visualisation and helps with the characterisation of focal lesions and abnormal structures in the CNS and liver in patients with known or highly suspected pathology.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08CA06
V - Various
V08 - Contrast media
V08C - Magnetic resonance imaging contrast media
V08CA - Paramagnetic contrast media
V08CA06 - Gadoversetamide
Gadoversetamide (0.1 mmol/kg) is eliminated primarily in the urine with 95.5 + or - 17.4% (mean + or - SD) of the administered dose eliminated by 24 hours. Animal data demonstrated that insignificant levels of 153Gd-labeled gadoversetamide are eliminated via the feces. In experimentally induced anephria in the rat, hepatobiliary excretion did not significantly compensate for the absence of urinary elimination. The renal and plasma clearance rates of gadoversetamide in normal subjects are similar (69 + or - 15.4 and 72 + or - 16.3 mL/hr/kg, respectively) indicating that the drug is cleared through the kidneys via glomerular filtration. Within the studied dose range (0.1 to 0.7 mmol/kg), the kinetics of gadoversetamide appear to be linear.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
The volume of distribution at steady state of gadoversetamide in normal subjects is 162 + or - 25 mL/kg, roughly equivalent to that of extracellular water.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
Radiolabeled gadoversetamide ((153)Gd) was excreted in the milk of lactating rats receiving a single intravenous dose of 0.1 mmol/kg.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
Optimark injection does not cross the intact blood brain barrier, and, therefore, does not accumulate in the normal brain or in lesions that may have a normal blood-brain barrier (eg, cysts, mature post-operative scars, etc.). However, disruption of the blood-brain barrier or abnormal vascularity allows accumulation of Optimark injection in the extravascular spaces of lesions such as neoplasms, abscesses, and subacute infarcts. The pharmacokinetic parameters of Optimark injection in various lesions are not known.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
For more Absorption, Distribution and Excretion (Complete) data for GADOVERSETAMIDE (6 total), please visit the HSDB record page.
Gadoversetamide is not metabolized.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a
The pharmacokinetics of intravenously administered gadoversetamide in normal subjects conforms to a two-compartment open-model with mean distribution and elimination half-lives (reported as mean + or - SD) of about 13.3 + or - 6.8 and 103.6 + or - 19.5 minutes.
NIH; DailyMed. Current Medication Information for Optimark (Gadoversetamide) Injection, Solution (Revised: October 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20372fd0-cd3c-47b2-bfc1-f5b8bc03ec0a