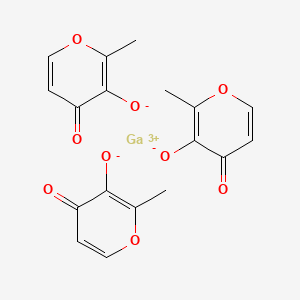

1. 4h-pyran-4-one, 3-hydroxy-2-methyl-, Gallium Salt (3:1)
2. Tris(3-hydroxy-2-methyl-4h-pyran-4-onato)gallium(iii)
3. Tris(maltolato)gallium(iii)
1. 108560-70-9
2. Gallium Tris(2-methyl-4-oxo-4h-pyran-3-olate)
3. 17lei49c2g
4. Gallium Maltolate [inci]
5. Gallium;2-methyl-4-oxopyran-3-olate
6. Db05420
7. B2699-054157
8. 3-bis[(2-methyl-4-oxopyran-3-yl)oxy]gallanyloxy-2-methylpyran-4-one
9. Gallium, Tris(3-(hydroxy-.kappa.o)-2-methyl-4h-pyran-4-onato-.kappa.o4)-, (oc-6-21)-
| Molecular Weight | 445.0 g/mol |
|---|---|
| Molecular Formula | C18H15GaO9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 148 |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Investigated for use/treatment in bladder cancer, lymphoma (unspecified), multiple myeloma, paget's disease, and prostate cancer.
Gallium maltolate is a novel orally active formulation of gallium, a semi-metallic element that is a potent inhibitor of ribonucleotide reductase, an enzyme that promotes tumor growth.Gallium is also known to concentrate in malignant tumors and sites of infection, and appears to favorably impact calcium deposition, making bones more resistant to degradation caused by cancer metastasis. Titan's novel product, gallium maltolate, may significantly expand the therapeutic potential of gallium by providing the advantages of enhanced bioavailablity, a potentially improved therapeutic profile and ease of administration.