
1. Apo Gemfibrozil
2. Apo-gemfibrozil
3. Apogemfibrozil
4. Ausgem
5. Bayvit, Gemfibrozilo
6. Bolutol
7. Chem Mart Gemfibrozil
8. Ci 719
9. Ci-719
10. Ci719
11. Dbl Gemfibrozil
12. Decrelip
13. Gemfi 1a Pharma
14. Gemfibrosil
15. Gemfibrozil, Genrx
16. Gemfibrozil, Healthsense
17. Gemfibrozil, Sbpa
18. Gemfibrozilo Bayvit
19. Gemfibrozilo Bexal
20. Gemfibrozilo Ur
21. Gemhexal
22. Gen Gemfibrozil
23. Gen-gemfibrozil
24. Gengemfibrozil
25. Genrx Gemfibrozil
26. Healthsense Gemfibrozil
27. Jezil
28. Lipazil
29. Lipox Gemfi
30. Lipur
31. Litarek
32. Lopid
33. Lopid R
34. Novo Gemfibrozil
35. Novo-gemfibrozil
36. Nu Gemfibrozil
37. Nu-gemfibrozil
38. Nugemfibrozil
39. Pilder
40. Pms Gemfibrozil
41. Pms-gemfibrozil
42. Sbpa Gemfibrozil
43. Terry White Chemists Gemfibrozil
44. Trialmin
1. 25812-30-0
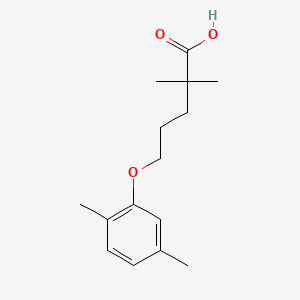
2. 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic Acid
3. Lopid
4. Jezil
5. Decrelip
6. Lipur
7. Ci-719
8. Gemfibrozilo
9. Gemfibrozilum
10. Pentanoic Acid, 5-(2,5-dimethylphenoxy)-2,2-dimethyl-
11. Gen-fibro
12. 2,2-dimethyl-5-(2,5-xylyloxy)valeric Acid
13. 2,2-dimethyl-5-(2,5-xylyloxy)valeriansaeure
14. 2,2-dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure
15. Gemfibrozil (lopid)
16. Bolutol
17. Apo-gemfibrozil
18. Cholespid
19. Gemfibril
20. Gemfibromax
21. Hipolixan
22. Fibratol
23. Fibrocit
24. Gemlipid
25. Lipazil
26. Litarek
27. Mfcd00079335
28. Trialmin
29. Ausgem
30. Pilder
31. Chembl457
32. Valeric Acid, 2,2-dimethyl-5-(2,5-xylyloxy)-
33. Nsc-757024
34. Mls000028421
35. Chebi:5296
36. Renabrazin
37. Clearol
38. Elmogan
39. Fetinor
40. Gemnpid
41. Innogen
42. Ipolipid
43. Lanaterom
44. Lifibron
45. Lipigem
46. Lipizyl
47. Micolip
48. Normolip
49. Polyxit
50. Progemzal
51. Reducel
52. Regulip
53. Sinelip
54. Synbrozil
55. Taborcil
56. Tentroc
57. Brozil
58. Lipira
59. Gozid
60. Hidil
61. Gemd
62. Wl-gemfibrozil
63. Gevilon Uno
64. Low-lip
65. 5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoic Acid
66. Q8x02027x3
67. 5-[(2,5-dimethylphenyl)oxy]-2,2-dimethylpentanoic Acid
68. Gem-s
69. Ncgc00016794-09
70. Lipozid
71. Smr000058393
72. Cas-25812-30-0
73. Dsstox_cid_652
74. Dsstox_rid_75712
75. Dsstox_gsid_20652
76. Gemfibrozilum [inn-latin]
77. Gemfibrozilo [inn-spanish]
78. Innogem
79. Gemcor
80. Ci 719
81. Ccris 318
82. Lopid (tn)
83. Teva-a
84. Sr-01000000056
85. Einecs 247-280-2
86. Brn 1881200
87. Gemfibrozil (jan/usp/inn)
88. Unii-q8x02027x3
89. Hsdb 7735
90. Gemfibrozil,(s)
91. 4tx
92. Gemfibrozil [usan:usp:inn:ban]
93. Prestwick_637
94. 2,2-dimethyl-5-(2,5-dimethylphenoxy)pentanoic Acid
95. Dimethylpentanoic Acid
96. 5-(2,5-dimethyl-phenoxy)-2,2-dimethyl-pentanoic Acid
97. Spectrum_000825
98. Cpd000058393
99. Gemfibrozil [mi]
100. 114413-98-8
101. Opera_id_1658
102. Prestwick0_000214
103. Prestwick1_000214
104. Prestwick2_000214
105. Prestwick3_000214
106. Spectrum2_001097
107. Spectrum3_000440
108. Spectrum4_000562
109. Spectrum5_000750
110. Spectrum5_001991
111. Gemfibrozil [inn]
112. Gemfibrozil [jan]
113. Gemfibrozil [hsdb]
114. Gemfibrozil [iarc]
115. Gemfibrozil [inci]
116. Gemfibrozil [usan]
117. Gemfibrozil [vandf]
118. Schembl4813
119. Gemfibrozil [mart.]
120. Bspbio_000227
121. Bspbio_002060
122. Gemfibrozil [usp-rs]
123. Gemfibrozil [who-dd]
124. Kbiogr_000964
125. Kbioss_001305
126. Mls001055364
127. Mls006011850
128. Divk1c_000138
129. Spectrum1500313
130. Spbio_001174
131. Spbio_002148
132. Bpbio1_000251
133. Gtpl3439
134. Yssj5501
135. Gemfibrozil, Analytical Standard
136. Dtxsid0020652
137. Hms500g20
138. Kbio1_000138
139. Kbio2_001305
140. Kbio2_003873
141. Kbio2_006441
142. Kbio3_001280
143. Gemfibrozil [orange Book]
144. Gemfibrozil For System Suitability
145. Ninds_000138
146. Gemfibrozil [ep Monograph]
147. Hms1568l09
148. Hms1920b07
149. Hms2090k14
150. Hms2091h11
151. Hms2095l09
152. Hms2230h24
153. Hms3259m12
154. Hms3655c06
155. Hms3712l09
156. Pharmakon1600-10500313
157. Gemfibrozil [usp Monograph]
158. 5-(2,5-dimethylphenoxy)-2,2-
159. Bcp08437
160. Hy-b0258
161. Zinc1530641
162. Tox21_110613
163. Tox21_201997
164. Tox21_302784
165. A0g461
166. Bbl010807
167. Bdbm50110590
168. Ccg-40111
169. Dl-414
170. Nsc757024
171. S1729
172. Stk618740
173. Akos001606691
174. Tox21_110613_1
175. Ab03034
176. Ac-4225
177. Db01241
178. Ks-5192
179. Nc00565
180. Nsc 757024
181. Idi1_000138
182. Ncgc00016794-01
183. Ncgc00016794-02
184. Ncgc00016794-03
185. Ncgc00016794-04
186. Ncgc00016794-05
187. Ncgc00016794-06
188. Ncgc00016794-07
189. Ncgc00016794-08
190. Ncgc00016794-10
191. Ncgc00016794-11
192. Ncgc00016794-13
193. Ncgc00016794-14
194. Ncgc00022722-03
195. Ncgc00022722-04
196. Ncgc00022722-05
197. Ncgc00022722-06
198. Ncgc00022722-07
199. Ncgc00256601-01
200. Ncgc00259546-01
201. Sy052512
202. Gemfibrozil 100 Microg/ml In Acetonitrile
203. Sbi-0051391.p003
204. 2,2-dimethyl-5-(2,5-zlyloxy)valeric Acid
205. Ab00052003
206. Ft-0626641
207. Ft-0700924
208. G0368
209. Sw196802-3
210. C07020
211. D00334
212. D83091
213. Ab00052003-15
214. Ab00052003-16
215. Ab00052003_17
216. Ab00052003_18
217. A818037
218. Q384295
219. 2,2-dimethyl-5-(2,5-dimethylphenoxy)valeric Acid
220. Sr-01000000056-3
221. Sr-01000000056-4
222. Sr-01000000056-6
223. Sr-01000000056-7
224. W-107216
225. 2,2-dimethyl-5-(2,5-dimethylphenoxy)-pentanoic Acid
226. 2,2-dimethyl-5-(2,5-xylyloxy) Valeric Acid
227. Brd-k11129031-001-05-1
228. Gemfibrozil, British Pharmacopoeia (bp) Reference Standard
229. Gemfibrozil, European Pharmacopoeia (ep) Reference Standard
230. Gemfibrozil, United States Pharmacopeia (usp) Reference Standard
231. Gemfibrozil, Pharmaceutical Secondary Standard; Certified Reference Material
232. Gemfibrozil For System Suitability, European Pharmacopoeia (ep) Reference Standard
233. 2,2-dimethyl-5-(2,5-dimethylphenoxy)pentanoic Acid, 2,2-dimethyl-5-(2,5-xylyloxy)valeric Acid, 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic Aci
| Molecular Weight | 250.33 g/mol |
|---|---|
| Molecular Formula | C15H22O3 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 250.15689456 g/mol |
| Monoisotopic Mass | 250.15689456 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 273 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Gemfibrozil |
| PubMed Health | Gemfibrozil (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Gemfibrozil is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. In addition, each tablet contains the following inactive ingredients: calcium stearate, colloidal silicon dioxide, h... |
| Active Ingredient | Gemfibrozil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Impax Pharms; Teva; Apotex; Sun Pharm Inds; Northstar Hlthcare; Invagen Pharms; Hikma Pharms; Dava Pharms; Blu Caribe |
| 2 of 4 | |
|---|---|
| Drug Name | Lopid |
| PubMed Health | Gemfibrozil (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | LOPID (gemfibrozil tablets, USP) is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. Each tablet also contains calcium stearate, NF; candelilla wax, FCC; microcrystalline cellulo... |
| Active Ingredient | Gemfibrozil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Gemfibrozil |
| PubMed Health | Gemfibrozil (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Gemfibrozil is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. In addition, each tablet contains the following inactive ingredients: calcium stearate, colloidal silicon dioxide, h... |
| Active Ingredient | Gemfibrozil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Impax Pharms; Teva; Apotex; Sun Pharm Inds; Northstar Hlthcare; Invagen Pharms; Hikma Pharms; Dava Pharms; Blu Caribe |
| 4 of 4 | |
|---|---|
| Drug Name | Lopid |
| PubMed Health | Gemfibrozil (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | LOPID (gemfibrozil tablets, USP) is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. Each tablet also contains calcium stearate, NF; candelilla wax, FCC; microcrystalline cellulo... |
| Active Ingredient | Gemfibrozil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
Gemfibrozil is used to reduce the risk of developing coronary heart disease in patients with type IIb hyperlipoproteinemia without clinical evidence of coronary heart disease (primary prevention) who have an inadequate response to dietary management, weight loss, exercise, and drugs known to reduce LDL-cholesterol and increase HDL-cholesterol (e.g., bile acid sequestrants) and who have low HDL-cholesterol concentrations in addition to elevated LDL-cholesterol and triglycerides. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1733
In a randomized, double-blind five-year trial, ... the efficacy of simultaneously elevating serum levels of high-density lipoprotein (HDL) cholesterol and lowering levels of non-HDL cholesterol with gemfibrozil in reducing the risk of coronary heart disease /was examined/ in 4081 asymptomatic middle-aged men (40 to 55 years of age) with primary dyslipidemia (non-HDL cholesterol greater than or equal to 200 mg per deciliter [5.2 mmol per liter] in two consecutive pretreatment measurements). One group (2051 men) received 600 mg of gemfibrozil twice daily, and the other (2030 men) received placebo. Gemfibrozil caused a marked increase in HDL cholesterol and persistent reductions in serum levels of total, low-density lipoprotein (LDL), and non-HDL cholesterol and triglycerides. There were minimal changes in serum lipid levels in the placebo group. The cumulative rate of cardiac end points at five years was 27.3 per 1,000 in the gemfibrozil group and 41.4 per 1,000 in the placebo group--a reduction of 34.0 percent in the incidence of coronary heart disease (95 percent confidence interval, 8.2 to 52.6; P less than 0.02; two-tailed test). The decline in incidence in the gemfibrozil group became evident in the second year and continued throughout the study. There was no difference between the groups in the total death rate, nor did the treatment influence the cancer rates. ...
PMID:3313041 Frick MH et al; N Engl J Med 317 (20): 1237-45 (1987).
... To compare the effectiveness and safety of lipid-lowering therapy in patients with and without HIV infection /a/ retrospective cohort study /was conducted in/ 829 patients with HIV infection and 6941 patients without HIV infection beginning lipid-lowering therapy for elevated low-density lipoprotein cholesterol or triglyceride levels. Compared with patients without HIV infection, patients with HIV infection beginning statin therapy had smaller reductions in low-density lipoprotein cholesterol levels (25.6% vs. 28.3%; P = 0.001), which did not vary by antiretroviral therapy class. Patients with HIV infection beginning gemfibrozil therapy had substantially smaller reductions in triglyceride levels than patients without HIV infection (44.2% vs. 59.3%; P < 0.001), and reductions with gemfibrozil varied by antiretroviral therapy class (44.0% [P = 0.001] in patients receiving protease inhibitors only, 26.4% [P < 0.001] in patients receiving protease inhibitors and nonnucleoside reverse transcriptase inhibitors [NNRTIs], and 60.3% [P = 0.94] in patients receiving NNRTIs only). Rhabdomyolysis was diagnosed in 3 patients with HIV infection and 1 patient without HIV infection. No clinically recognized cases of myositis or myopathy were observed. The risk for laboratory adverse events was low (<5%), although it was increased in patients with HIV infection. Limitations: Laboratory measurements were not uniformly performed according to HIV status, and adequate fasting before lipoprotein testing could not be verified. Results may not be completely generalizable to uninsured persons, women, or certain racial or ethnic minorities. Dyslipidemia, particularly hypertriglyceridemia, is more difficult to treat in patients with HIV infection than in the general population. However, patients with HIV infection receiving NNRTI-based antiretroviral therapy and gemfibrozil had triglyceride responses similar to those in patients without HIV infection.
PMID:19258558 Silverberg MJ et al; Ann Intern Med 150 (5): 301-13 (2009).
Gemfibrozil is used as an adjunct to dietary therapy for the management of severe hypertriglyceridemia in patients at risk of developing pancreatitis (typically those with serum triglyceride concentrations exceeding 2000 mg/dL and elevated concentrations of VLDL and fasting chylomicrons) who do not respond adequately to dietary management. Gemfibrozil also may be used in patients with triglyceride concentrations of 1000-2000 mg/dL who have a history of pancreatitis or of recurrent abdominal pain typical of pancreatitis; however, efficacy of the drug in patients with type IV hyperlipoproteinemia and triglyceride concentrations less than 1000 mg/dL who exhibit type V patterns subsequent to dietary or alcoholic indiscretion has not been adequately studied. The manufacturer states that gemfibrozil is not indicated for use in patients with type I hyperlipoproteinemia who have elevated triglyceride and chylomicron concentrations but normal VLDL-cholesterol concentrations. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1732
For more Therapeutic Uses (Complete) data for Gemfibrozil (6 total), please visit the HSDB record page.
Adverse effects of gemfibrozil are infrequent and generally mild; however, because of the chemical, pharmacologic, and clinical similarities between clofibrate (no longer commercially available in the US) and gemfibrozil, the possibility that gemfibrozil may share the toxic potentials of clofibrate should be considered.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1735
The most frequent adverse effects of gemfibrozil involve the GI tract and occasionally may be severe enough to require discontinuance of the drug. Abdominal pain (and, in some instances, acute appendicitis), and epigastric pain or dyspepsia are common adverse GI effects reported with gemfibrozil. Nausea, vomiting, diarrhea, constipation, and flatulence occur less frequently; cholestatic jaundice also has been reported. Dry mouth, anorexia and/or weight loss, gas pain, pancreatitis, colitis, and heartburn have also been reported in patients receiving gemfibrozil but have not been directly attributed to the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1735
Headache, dizziness, drowsiness or somnolence, blurred vision, paresthesia, hypesthesia, taste perversion, peripheral neuritis, mental depression, and impotence and decreased libido have been reported in patients receiving gemfibrozil. Although a causal relationship has not been established, vertigo, syncope, insomnia, asthenia, chills, psychic problems, fatigue, confusion, and seizures have also occurred in patients receiving the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1735
Slight decreases in hemoglobin and hematocrit and in leukocyte count have occurred in a few patients receiving gemfibrozil; these levels stabilize during long-term administration. Eosinophilia has also been reported. The drug may also affect blood coagulation. Severe anemia, leukopenia, thrombocytopenia, and bone marrow hypoplasia reportedly have occurred rarely in patients receiving gemfibrozil. Therefore, the manufacturer recommends that blood cell counts be monitored periodically during the first 12 months of therapy.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1735
For more Drug Warnings (Complete) data for Gemfibrozil (19 total), please visit the HSDB record page.
Gemfibrozil is indicated to treat patients with Types IV and V hyperlipidemia who have elevated serum triglycerides (usually above 2000mg/dL), elevated VLDL cholesterol, fasting chylomicrons, are at risk of developing pancreatitis, and do not adequately respond to dietary restrictions. Gemfibrozil is also indicated to reduce the risk of developing coronary heart disease in patients with Type IIb hyperlipidemia without history or symptoms of coronary heart disease; who do not adequately respond to weight loss, diet, exercise, and other medications; and have low HDL, raised LDL, and raised triglycerides.
FDA Label
Gemfibrozil alters lipid metabolism to treat patients with hyperlipidemia. The duration of action requires twice daily dosing as the mean residence time of gemfibrozil is up to 9.6h in patients with chronic renal failure. Gemfibrozil has a wide therapeutic index as trials with twice the standard dose were not associated with severe side effects. Patients taking gemfibrozil may be at an increased risk of developing cholelithiasis and cholecystitis, as seen in patients taking [clofibrate].
Cytochrome P-450 CYP2C8 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C8. (See all compounds classified as Cytochrome P-450 CYP2C8 Inhibitors.)
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AB - Fibrates
C10AB04 - Gemfibrozil
Absorption
Gemfibrozil is absorbed from the gastrointestinal tract. In healthy volunteers, a 900mg oral dose of gemfibrozil has a Cmax of 4616g/mL with a Tmax of 2.21.1h. In patients with chronic renal failure, gemfibrozil has a Cmax of 13.811.1g/mL with a Tmax of 2.31.0h. In patients with liver disease, gemfibrozil has a Cmax of 23.010.3g/mL with a Tmax of 2.61.7h.
Route of Elimination
Approximately 70% of a dose of gemfibrozil is eliminated in the urine. The majority of a dose is eliminated as a glucuronide conjugate and <2% is elimiinated as the unmetabolized drug. 6% of a dose is eliminated in the feces. In healthy volunteers, 0.02-0.15% of a dose was detected in the urine as unmetabolized gemfibrozil, with 7-14% detected as conjugated metabolites. In patients with renal failure, trace amounts of unmetabolized gemfibrozil is present in the urine, with 0.5-9.8% detected as conjugated metabolites. In patients with liver disease, 0.1-0.2% of a dose was detected in the urine as unmetabolized gemfibrozil, with 25-50% detected as conjugated metabolites.
Volume of Distribution
The volume of distribution of gemfibrozil is estimated to be 0.8L/kg.
Clearance
The clearance of gemfibrozil is estimated to be 6.0L/h.
Studies in monkeys indicate that gemfibrozil crosses the placenta.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
About 95% of gemfibrozil is protein bound. In vitro at concentrations of 0.1-12 ug/mL, 97% of gemfibrozil is bound to 4% human serum albumin; the major metabolite of gemfibrozil (metabolite III) has no effect on the binding capacity of gemfibrozil.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
In animals, maximum tissue concentrations of gemfibrozil were reached 1 hour after administration of a single dose, and highest concentrations occurred in liver and kidneys.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
Gemfibrozil is rapidly and completely absorbed from the GI tract. The relative bioavailability of gemfibrozil capsules compared with an oral solution of the drug is 97%. The drug undergoes enterohepatic circulation. Plasma gemfibrozil concentrations show marked interindividual variability but tend to increase proportionally with increasing dose. Plasma concentrations of the drug do not appear to correlate with therapeutic response. Following single or multiple oral doses of gemfibrozil, peak plasma concentrations of the drug occur within 1-2 hours. Following oral administration of a single 800-mg dose in healthy adults in one study, mean peak plasma gemfibrozil concentrations of 33 ug/mL occurred 1-2 hours after ingestion. Following oral administration of multiple doses of the drug (600 mg twice daily) in healthy adults in another study, mean peak plasma concentrations of the drug were 16-23 ug/mL about 1-2 hours after a dose.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
For more Absorption, Distribution and Excretion (Complete) data for Gemfibrozil (9 total), please visit the HSDB record page.
Gemfibrozil undergoes hydroxylation at the 5'-methyl and 4' positions to form the M1 and M2 metaolites respectively. Gemfibrozil also undergoes O-glucuronidation to form gemfibrozil 1-beta glucuronide, an inhibitor of CYP2C8. This O-glucuronidation is primarily mediated by UGT2B7, but also by UGT1A1, UGT1A3, UGT1A9, UGT2B4, UGT2B17.
Gemfibrozil is biotransformed extensively following oral administration. A major pathway of gemfibrozil metabolism is via glucuronidation. Following a single oral administration of 450 mg (6 mg/kg bw est) gemfibrozil to six male subjects, gemfibrozil glucuronide represented approximately 50% of the total urinary metabolites (32% of the dose) recovered within 24 hr. Very similar results had been obtained in /another/ ... study, in which 31% of the dose was recovered as urinary gemfibrozil glucuronide over 0-48 hr. Among metabolites resulting from phase I biotransformation, 5-(5-carboxy-2-methylphenoxy)-2,2-dimethyl pentanoic acid (M3) was the major metabolite recovered. ... A 24-hr urine collection contained both free and conjugated M3 at approximately 15% and 5% of the total dose, respectively, while, in /another/ study, free and conjugated M3 represented approximately 7% and 5% of the recovered radiactivity, respectively. Other minor metabolites identified were the 5-hydroxymethyl derivative (M2, an intermediate in the pathway to M3), a 4-hydroxy derivative (M1) and a 2- hydroxymethyl derivative (M4). In aggregate, urinary and fecal excretion of radioactivity accounted for 66% and 6%, respectively, of the elimination of orally administered gemfibrozil over five days.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 431 (1996)
The exact metabolic fate of gemfibrozil has not been fully elucidated, but the drug appears to be metabolized in the liver to 4 major metabolites produced via 3 metabolic pathways. Gemfibrozil undergoes hydroxylation of the m-methyl group to the corresponding benzyl alcohol derivative (metabolite II), which is rapidly oxidized to a benzoic acid metabolite (metabolite III, 3-[(4-carboxy-4-methylpentyl)oxy]-4-methylbenzoic acid), the major metabolite. The drug also undergoes hydroxylation of the aromatic ring to produce a phenol derivative (metabolite I) which is probably further metabolized to a compound that is phenolic but has no intact carboxylic acid function (metabolite IV). Metabolite I is pharmacologically active. The drug and its metabolites also undergo conjugation.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
The roles of multidrug resistance-associated protein (Mrp) 2 deficiency and Mrp3 up-regulation were evaluated on the metabolism and disposition of gemfibrozil. Results from in vitro studies in microsomes showed that the hepatic intrinsic clearance (CLint) for the oxidative metabolism of gemfibrozil was slightly higher (1.5-fold) in male TR- rats, which are deficient in Mrp2, than in wild-type Wistar rats, whereas CLint for glucuronidation was similar in both strains. The biliary excretion of intravenously administered [14C]gemfibrozil was significantly impaired in TR-) rats compared with Wistar rats (22 versus 93% of the dose excreted as the acyl glucuronides over 72 h). Additionally, the extent of urinary excretion of radioactivity was much higher in TR- than in Wistar rats (78 versus 2.6% of the dose). There were complex time-dependent changes in the total radioactivity levels and metabolite profiles in plasma, liver and kidney, some of which appeared to be related to the up-regulation of Mrp3. Overall, it was demonstrated that alterations in the expression of the transporters Mrp2 and Mrp3 significantly affected the excretion as well as the secondary metabolism and distribution of (14)Cgemfibrozil.
PMID:14555339 Kim MS et al; Xenobiotica 33 (10): 1027-42 (2003).
... (14)C-Gemfibrozil was administered orally to rats at a dose of 2000 mg/kg. At various time points, radioactivity in urine was analyzed by liquid scintillation spectrometry, high-pressure liquid chromatography, liquid chromatography/mass spectrometry, gas chromatography/mass spectroscopy, and nuclear magnetic resonance. Nine metabolites of gemfibrozil were identified, some that have not been reported previously. Although the majority of metabolites were glucuronidated, some nonglucuronidated metabolites were identified in urine, including a diol metabolite (both ring methyls hydroxylated), and the product of its further metabolism, the acid-alcohol derivative (ortho ring methyl hydroxylated, meta ring methyl completely oxidized to the acid). Hydroxylation of the aromatic ring also was a common pathway for gemfibrozil metabolism, leading to the production of two phenolic metabolites, only one of which was detected in the urine in the nonconjugated or free form. Also of interest was the finding that both acyl and ether glucuronides were produced, including both glucuronide forms of the same metabolite (e. g., 1-O-GlcUA, 5'-COOH-gemfibrozil, and 5'-COO-GlcUA-gemfibrozil); the positions and functionality of the glucuronide conjugates were identified using base hydrolysis or glucuronidase treatment, in combination with liquid chromatography/MS and nuclear magnetic resonance.
PMID:9884324 Thomas BF et al; Drug Metab Dispos 27 (1): 147-57 (1999).
Gemfibrozil has known human metabolites that include (2S,3S,4S,5R)-6-[5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Gemfibrozil has a plasma half-life of 1.5 hours. In patients with renal failure the half life is 2.4h and in patients with liver disease the half life is 2.1h.
The elimination half-life of gemfibrozil is about 1.5 hours after a single dose and 1.3-1.5 hours after multiple doses in individuals with normal renal function.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1738
The disposition of the lipid-lowering drug gemfibrozil was studied in patients with either renal (n = 8) or hepatic disease (n = 8) and compared to those of healthy volunteers (n = 6). ... Following oral administration of 900 mg gemfibrozil ... the elimination half-life of the drug was 1.5 hr in controls, 2.4 hr in renal failure, and 2.1 hr in liver disease.
PMID:2381138 Knauf H et al; Klin Wochenschr 68 (13): 692-8 (1990).
Gemfibrozil activates peroxisome proliferator-activated receptor- (PPAR), which alters lipid metabolism. This activation leads to increased HDL, apo AI, apo AII, lipoprotein lipase (LPL), inhibition of apo B synthesis, peripheral lipolysis, decreased removal of free fatty acids by the liver, and increased clearance of apoB. Upregulated LPL reduces plasma triglyceride levels. Decreased hepatic removal of fatty acids decreases the production of triglycerides. The effects on apoB synthesis and clearance decrease VLDL production which also reduce plasma triglyceride levels. Gemfibrozil's glucuronide metabolite is also an inhibitor of CYP2C8.
The present study underlines the importance of PI3K in mediating the anti-inflammatory effect of gemfibrozil, a prescribed lipid-lowering drug for humans, in mouse microglia. Gemfibrozil inhibited LPS-induced expression of inducible NO synthase (iNOS) and proinflammatory cytokines in mouse BV-2 microglial cells and primary microglia. By overexpressing wild-type and dominant-negative constructs of peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in microglial cells and isolating primary microglia from PPAR-alpha-/- mice, we have demonstrated that gemfibrozil inhibits the activation of microglia independent of PPAR-alpha. Interestingly, gemfibrozil induced the activation of p85alpha-associated PI3K (p110beta but not p110alpha) and inhibition of that PI3K by either chemical inhibitors or dominant-negative mutants abrogated the inhibitory effect of gemfibrozil. Conversely, overexpression of the constitutively active mutant of p110 enhanced the inhibitory effect of gemfibrozil on LPS-induced expression of proinflammatory molecules. Similarly, gemfibrozil also inhibited fibrillar amyloid beta (Abeta)-, prion peptide (PrP)-, dsRNA (poly IC)-, HIV-1 Tat-, and 1-methyl-4-phenylpyridinium (MPP+)-, but not IFN-gamma-, induced microglial expression of iNOS. Inhibition of PI3K also abolished the inhibitory effect of gemfibrozil on Abeta-, PrP-, poly IC-, Tat-, and MPP+-induced microglial expression of iNOS. Involvement of NF-kappaB activation in LPS-, Abeta-, PrP-, poly IC-, Tat-, and MPP+-, but not IFN-gamma-, induced microglial expression of iNOS and stimulation of IkappaBalpha expression and inhibition of NF-kappaB activation by gemfibrozil via the PI3K pathway suggests that gemfibrozil inhibits the activation of NF-kappaB and the expression of proinflammatory molecules in microglia via PI3K-mediated up-regulation of IkappaBalpha.
PMID:17785853 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2604815 Jana M et al; J Immunol 179 (6): 4142-52 (2007).
... After 2-4 months of gemfibrozil therapy in patients with type IIa, IIb, IV, or V hyperlipoproteinemia, HDL-apoprotein A-I (HDL-apoA-I) may be either unchanged or increased and HDL-apoprotein A-II (HDL-apoA-II) is increased. Unlike niacin, gemfibrozil has not been shown to reduce HDL2 catabolism. Gemfibrozil has been reported to increase the synthetic rates of HDL-apoA-I and HDL-apoA-II in patients with type V hyperlipoproteinemia. The HDL2 subfraction is increased to a greater degree than the HDL3 subfraction during gemfibrozil therapy. Low HDL2 concentrations have been reported to correlate with increased coronary heart disease. Gemfibrozil increases serum reserve cholesterol binding capacity (SRCBC), the capacity of serum to solubilize additional cholesterol, by about 60%. SRCBC exists in a subclass of HDL.31 In patients with types IIa, IIb, or IV hyperlipoproteinemia, serum concentrations of apoprotein B usually decrease during gemfibrozil therapy; decreases in apoprotein B follow changes in LDL-cholesterol. However, in another study in patients with primary hypertriglyceridemia associated with coronary heart disease (CHD), LDL-apoprotein B increased in a few patients. In patients with type V hyperlipoproteinemia, apoprotein B concentration has been reported to increase during gemfibrozil therapy.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1737
In humans, gemfibrozil inhibits lipolysis of fat in adipose tissue and decreases the hepatic uptake of plasma free fatty acids (i.e., free fatty acid turnover is decreased), thereby reducing hepatic triglyceride production (triglyceride turnover rate is decreased). The drug also reportedly inhibits production and increases clearance of VLDL-apoprotein B (VLDL-apoB), leading to a decrease in VLDL-triglyceride production, enhanced clearance of VLDL-triglyceride, and, subsequently, a decrease in serum triglyceride concentrations. The increase in serum total LDL concentration that may occur with gemfibrozil may be caused by a decrease in the catabolic rate of LDL, possibly secondary to an effect(s) of the drug on hepatic metabolism of LDL, and/or by an increase in the catabolic rate of VLDL-cholesterol. In animals, gemfibrozil reduces incorporation of long-chain fatty acids into newly formed triglycerides and inhibits basal, norepinephrine-induced, isoproterenol-stimulated, and cyclic adenosine-3',5'-monophosphate (AMP)-stimulated lipolysis of adipose tissue. It has been proposed that this reduction in adipose tissue lipolysis may be a mechanism for decreased serum triglyceride concentrations; however, it is unlikely that the drug's antilipemic effect in humans results from this mechanism of action.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1737