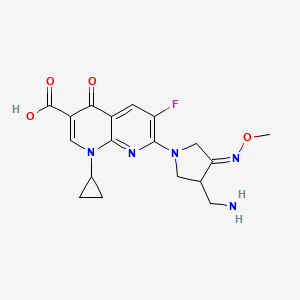

1. 7-(3-aminomethyl-4-methoxyimino-pyrrolidine-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-(1,8)-naphthyridine-3-carboxylic Acid
2. Factive
3. Gemifloxacin Mesylate
4. Lb 20304
5. Lb-20304
6. Lb20304
7. Sb 265805
8. Sb-265805
9. Sb265805
1. Gemifioxacin
2. 175463-14-6
3. Gemifloxacin [inn]
4. Gemifloxacin Mesilate
5. Factiv
6. Gemifloxacin (inn)
7. Lb-20304
8. 7-(3-aminomethyl-4-methoxyimino-pyrrolidine-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-(1,8)-naphthyridine-3-carboxylic Acid
9. Chembl430
10. Okr68y0e4t
11. Chebi:101853
12. Sb-265805
13. (z)-7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic Acid
14. 7-[(4z)-3-(aminomethyl)-4-methoxyimino-pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic Acid
15. 7-[3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic Acid
16. Factiv (tn)
17. Lb 20304
18. Unii-okr68y0e4t
19. Sr-05000001958
20. Hsdb 8027
21. 7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic Acid
22. Gemifloxacin [mi]
23. Gemifloxacin [vandf]
24. Bspbio_002308
25. 204519-64-2
26. Gemifloxacin [who-dd]
27. Schembl136633
28. Dtxsid3048495
29. Schembl13414391
30. Bdbm50178917
31. Akos015907681
32. Am84619
33. Db01155
34. Ncgc00178711-01
35. Ncgc00178711-06
36. 1,8-naphthyridine-3-carboxylic Acid, 7-(3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-
37. 7-(3-aminomethyl)-4-methoxyimino-pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic Acid
38. Sbi-0206906.p001
39. Hy-116229
40. Cs-0064428
41. D08012
42. Ab01563334_01
43. 463g146
44. A812090
45. Sr-05000001958-2
46. Brd-a40787240-066-02-0
47. Brd-a40787240-066-03-8
48. (+/-)-7-(3-(aminomethyl)-4-oxo-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic Acid, 7(sup 4)-(z)-(o)-methyloxime)
49. (s,z)-7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic Acid
50. 1,4-dihydro-1-cyclopropyl-4-oxo-6-fluoro-7-[3-(aminomethyl)-4-(methoxyimino)pyrrolidine-1-yl]-1,8-naphthyridine-3-carboxylic Acid
51. 1,4-dihydro-6-fluoro-1-cyclopropyl-4-oxo-7-[(3e)-4-(aminomethyl)-3-(methoxyimino)pyrrolidine-1-yl]-1,8-naphthyridine-3-carboxylic Acid
52. 1,8-naphthyridine-3-carboxylic Acid, 7-(3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4- Oxo-
53. 1,8-naphthyridine-3-carboxylic Acid, 7-[(4z)-3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-
54. 7-[(4z)-3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic Acid
55. 7-[(4z)-3-(aminomethyl)-4-methoxyimino-pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic Acid;gemifloxacin
56. 7-{3-aminomethyl-4-[(z)-methoxyimino]-pyrrolidin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic Acid
57. Gemifloxacin; 7-(3-aminomethyl)-4-methoxyimino-pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1, 4-dihydro-[1, 8]naphthyridine-3-carboxylic Acid; 210353-56-3
| Molecular Weight | 389.4 g/mol |
|---|---|
| Molecular Formula | C18H20FN5O4 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 389.14993230 g/mol |
| Monoisotopic Mass | 389.14993230 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 725 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Factive |
| PubMed Health | Gemifloxacin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chem... |
| Active Ingredient | Gemifloxacin mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 320mg base |
| Market Status | Prescription |
| Company | Lg Life Sciences |
| 2 of 2 | |
|---|---|
| Drug Name | Factive |
| PubMed Health | Gemifloxacin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chem... |
| Active Ingredient | Gemifloxacin mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 320mg base |
| Market Status | Prescription |
| Company | Lg Life Sciences |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Gemifloxacin is used for the treatment of acute bacterial exacerbation of chronic bronchitis caused by susceptible Streptococcus pneumoniae, Haemophilus influenzae, H. parainfluenzae, or Moraxella catarrhalis. /Included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 381
Gemifloxacin is used for the treatment of community-acquired pneumonia (CAP) of mild to moderate severity caused by susceptible S. pneumoniae (including multidrug-resistant strains), H. influenzae, M. catarrhalis, Mycoplasma pneumoniae, Chlamydophila pneumoniae (formerly Chlamydia pneumoniae), or Klebsiella pneumoniae. /Included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 381
/BOXED WARNING/ WARNING: Fluoroquinolones, including Factive, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants.
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=63f60426-e27f-4258-9bfc-b4a15ebdca83
/BOXED WARNING/ WARNING: Fluoroquinolones, including Factive, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid Factive in patients with known history of myasthenia gravis
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=63f60426-e27f-4258-9bfc-b4a15ebdca83
Fluoroquinolones, including gemifloxacin, cause arthropathy and osteochondrosis in immature animals of various species. The relevance of these adverse effects in immature animals to use in humans is unknown.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 382
Fluoroquinolones, including gemifloxacin, are associated with increased risk of tendinitis and tendon rupture in all age groups. This risk is further increased in older adults (usually those older than 60 years of age), individuals receiving concomitant corticosteroids, and kidney, heart, or lung transplant recipients. Other factors that may independently increase risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients receiving fluoroquinolones who did not have any of these risk factors. Fluoroquinolone-associated tendinitis and tendon rupture most frequently involve the Achilles tendon and may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (shoulder), hand, biceps, thumb, and other tendon sites also reported. Tendon rupture can occur during or following fluoroquinolone therapy and has been reported up to several months after completion of therapy. Advise patients to rest and refrain from exercise and contact a clinician at the first sign of tendinitis or tendon rupture (e.g., pain, swelling, or inflammation of a tendon or weakness or inability to use a joint). Discontinue gemifloxacin if pain, swelling, inflammation, or rupture of a tendon occurs.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 382
For more Drug Warnings (Complete) data for Gemifloxacin (21 total), please visit the HSDB record page.
For the treatment of bacterial infection caused by susceptible strains such as S. pneumoniae, H. influenzae, H. parainfluenzae, or M. catarrhalis, S. pneumoniae (including multi-drug resistant strains [MDRSP]), M. pneumoniae, C. pneumoniae, or K. pneumoniae.
FDA Label
Gemifloxacin is a quinolone/fluoroquinolone antibiotic. Gemifloxacin is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase, which allows the untwisting required to replicate one DNA double helix into two. Notably the drug has 100 times higher affinity for bacterial DNA gyrase than for mammalian. Gemifloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MA - Fluoroquinolones
J01MA15 - Gemifloxacin
Absorption
Rapidly absorbed from the gastrointestinal tract. The absolute bioavailability averages approximately 71%.
Route of Elimination
Gemifloxacin and its metabolites are excreted via dual routes of excretion.Following oral administration of gemifloxacin to healthy subjects, a mean ( SD) of 61 9.5% of the dose was excreted in the feces and 36 9.3% in the urine as unchanged drug and metabolites. The mean ( SD) renal clearance following repeat doses of 320 mg was approximately 11.6 3.9 L/hr (range 4.6-6 L/hr), which indicates active secretion is involved in the renal excretion of gemifloxacin.
Volume of Distribution
1.66 to 12.12 L/kg
Clearance
renal cl=11.6+/- 3.9 L/hr [Healthy subjects receiving repeat doses of 320 mg orally]
Gemifloxacin, given as an oral tablet, is rapidly absorbed from the gastrointestinal tract. Peak plasma concentrations of gemifloxacin were observed between 0.5 and 2 hours following oral tablet administration and the absolute bioavailability of the 320 mg tablet averaged approximately 71% (95% CI 60%-84%). Following repeat oral doses of 320 mg to healthy subjects, the mean + or - SD maximal gemifloxacin plasma concentrations (Cmax) and systemic drug exposure (AUC (0-24)) were 1.61 + or - 0.51 ug/mL (range 0.70-2.62 ug/mL) and 9.93 + or - 3.07 ug.hr/mL (range 4.71-20.1 ug.hr/mL), respectively. In patients with respiratory and urinary tract infections (n=1423), similar estimates of systemic drug exposure were determined using a population pharmacokinetics analysis (geometric mean AUC (0-24), 8.36 ug.hr/mL; range 3.2 -47.7 ug.hr/mL).
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=54c4b48b-cad7-4889-808f-f741352a2c97
In vitro binding of gemifloxacin to plasma proteins in healthy subjects is approximately 60 to 70% and is concentration independent. After repeated doses, the in vivo plasma protein binding in healthy elderly and young subjects ranged from 55% to 73% and was unaffected by age. Renal impairment does not significantly affect the protein binding of gemifloxacin. The blood-to-plasma concentration ratio of gemifloxacin was 1.2:1. The geometric mean for Vdss/F is 4.18 L/kg (range, 1.66 - 12.12 L/kg). Gemifloxacin is widely distributed throughout the body after oral administration. Concentrations of gemifloxacin in bronchoalveolar lavage fluid exceed those in the plasma. Gemifloxacin penetrates well into lung tissue and fluids.
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=54c4b48b-cad7-4889-808f-f741352a2c97
Gemifloxacin and its metabolites are excreted via dual routes of excretion. Following oral administration of gemifloxacin to healthy subjects, a mean (+ or - SD) of 61 + or - 9.5% of the dose was excreted in the feces and 36 + or - 9.3% in the urine as unchanged drug and metabolites. The mean (+ or - SD) renal clearance following repeat doses of 320 mg was approximately 11.6 + or - 3.9 L/hr (range 4.6-17.6 L/hr), which indicates active secretion is involved in the renal excretion of gemifloxacin. The mean (+ or - SD) plasma elimination half-life at steady state following 320 mg to healthy subjects was approximately 7 + or - 2 hours (range 4-12 hours).
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=54c4b48b-cad7-4889-808f-f741352a2c97
Gemifloxacin is metabolized to a limited extent by the liver. All metabolites formed are minor (<10% of the administered oral dose); the principal ones are N-acetyl gemifloxacin, the E-isomer of gemifloxacin and the carbamyl glucuronide of gemifloxacin.
Gemifloxacin is metabolized to a limited extent by the liver. The unchanged compound is the predominant drug-related component detected in plasma (approximately 65%) up to 4 hours after dosing. All metabolites formed are minor (<10% of the administered oral dose); the principal ones are N-acetyl gemifloxacin, the E-isomer of gemifloxacin and the carbamyl glucuronide of gemifloxacin. Cytochrome P450 enzymes do not play an important role in gemifloxacin metabolism, and the metabolic activity of these enzymes is not significantly inhibited by gemifloxacin.
US Natl Inst Health; DailyMed. Current Medication Information for FACTIVE (gemifloxacin mesylate) tablet, (November 2011). Available from, as of February 28, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=54c4b48b-cad7-4889-808f-f741352a2c97
7 (± 2) hours
Gemifloxacin has an elimination half life of about 7 hours.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 384
The bactericidal action of gemifloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are required for bacterial DNA replication, transcription, repair, and recombination.
Like other fluoroquinolone anti-infective agents, gemifloxacin inhibits DNA synthesis in susceptible organisms via inhibition of type II DNA topoisomerases (DNA gyrase, topoisomerase IV). However, unlike many other fluoroquinolones, gemifloxacin targets both DNA gyrase and topoisomerase IV in susceptible S. pneumoniae. Although cross-resistance can occur between gemifloxacin and other fluoroquinolones, gemifloxacin may be active against some strains of S. pneumoniae resistant to ciprofloxacin and other fluoroquinolones.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 384
The fluroquinolone gemifloxacin was examined for its capacity to modulate secretion of cytokines by human monocytes stimulated with lipopolysaccharide (LPS). Monocytes from six male and two female healthy volunteers were stimulated with LPS, exposed to gemifloxacin and the amounts of secreted IL-1 alpha, IL-1 beta, IL-6, IL-10 and TNF-alpha measured at 3, 6 and 24 h. The results revealed that LPS alone increased secretion of each cytokine significantly. Treatment of the LPS-stimulated monocytes with gemifloxacin resulted in a significant inhibition (p < 0.01) of secretion of each of the cytokines from monocytes of the eight volunteers. Nuclear extracts of the human monocyte cell line, THP-1, were used in the electrophoretic mobility shift assay to determine whether gemifloxacin affects nuclear factor-kappa B (NF-kappa B) activation. In addition, RNA from THP-1 cells was used in Northern blots to determine whether inhibition of secretion of IL-1 beta and TNF-alpha by gemifloxacin occurred at the transcription or translation level. Whereas LPS induced a rapid increase in NF-kappa B activation, gemifloxacin alone did not. Gemifloxacin did not affect the kinetics or decrease the extent of activation. Northern blots indicated that the inhibitory activity of gemifloxacin occurred post-transcription. Thus, gemifloxacin may modulate the immune response by altering secretion of cytokines by human monocytes. Although the concentrations of gemifloxacin used were higher than those observed in the serum of human volunteers treated with the dose under clinical development, it should be taken into consideration that concentrations at tissue and intracellular levels may be considerably higher than serum concentrations.
PMID:15008941 Araujo F et al; Clin Microbiol Infect 10 (3): 213-9 (2004)
Fluoroquinolones prolong the QT interval by blocking voltage-gated potassium channels, especially the rapid component of the delayed rectifier potassium current I(Kr), expressed by HERG (the human ether-a-go-go-related gene). According to the available case reports and clinical studies, moxifloxacin carries the greatest risk of QT prolongation from all available quinolones in clinical practice and it should be used with caution in patients with predisposing factors for Torsades de pointes (TdP).
PMID:22156660 Briasoulis A et al; Cardiology 120 (2): 103-10 (2011)