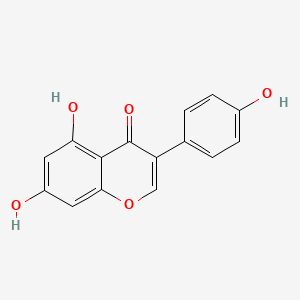

1. Genestein
1. 446-72-0
2. Prunetol
3. 4',5,7-trihydroxyisoflavone
4. Genisterin
5. Genisteol
6. Sophoricol
7. 5,7-dihydroxy-3-(4-hydroxyphenyl)-4h-chromen-4-one
8. 5,7,4'-trihydroxyisoflavone
9. Bonistein
10. Differenol A
11. 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one
12. Npi 031l
13. 5,7-dihydroxy-3-(4-hydroxyphenyl)-4h-1-benzopyran-4-one
14. 4h-1-benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-
15. C.i. 75610
16. Sipi 807-1
17. Mfcd00016952
18. 5,7-dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone
19. Nsc 36586
20. Genistein [usan]
21. Nsc36586
22. Chembl44
23. Nsc-36586
24. Bio 300
25. Isoflavone, 4',5,7-trihydroxy-
26. Mls000738127
27. Dh2m523p0h
28. Npi-031l
29. Genestein
30. Chebi:28088
31. Bio-300
32. Sipi-807-1
33. Genistein (usan)
34. Fw-635i-2
35. Gen
36. Ncgc00015479-09
37. Dsstox_cid_2308
38. Dsstox_rid_76542
39. Dsstox_gsid_22308
40. Cas-446-72-0
41. Lactoferrin-genistein
42. Ccris 7675
43. 4',5, 7-trihydroxyisoflavone
44. Sr-01000075498
45. Einecs 207-174-9
46. Brn 0263823
47. Unii-dh2m523p0h
48. Sto514
49. Hsdb 7475
50. Pti G4660
51. Pti-g4660
52. Sipi-9764-i
53. 3kgt
54. 3kgu
55. Genistein, 8
56. Genistein,(s)
57. Pti-g 4660
58. Tnp00151
59. Genistein (flavonoid)
60. Spectrum_000320
61. Tocris-1110
62. 1x7r
63. 2qa8
64. Genistein 85% Hplc
65. Genistein [inn]
66. Specplus_000305
67. Genistein [mi]
68. Genistein [hsdb]
69. Genistein [inci]
70. Spectrum2_000638
71. Spectrum3_000678
72. Spectrum4_001543
73. Spectrum5_000106
74. Lopac-g-6649
75. 4',7-trihydroxyisoflavone
76. Genistein [mart.]
77. Molmap_000022
78. Upcmld-dp096
79. G 6649
80. Genistein [usp-rs]
81. Genistein [who-dd]
82. 4,5,7-trihydroxyisoflavone
83. Isoflavone,5,7-trihydroxy-
84. Lopac0_000520
85. Oprea1_224620
86. Oprea1_437815
87. Schembl19166
88. Bspbio_002375
89. Kbiogr_002006
90. Kbiogr_002564
91. Kbioss_000800
92. Kbioss_002573
93. Npi031l
94. Spectrum210296
95. 5-18-04-00594 (beilstein Handbook Reference)
96. Bidd:er0113
97. Divk1c_006401
98. Genistein, Analytical Standard
99. Spbio_000636
100. 4',5,7-trihydroxy-isoflavone
101. Gtpl2826
102. Megxp0_000568
103. 4,5,7-trihydroxy Iso-flavone
104. Dtxsid5022308
105. Upcmld-dp096:001
106. Acon1_001065
107. Bdbm19459
108. Cid_5280961
109. Kbio1_001345
110. Kbio2_000800
111. Kbio2_002564
112. Kbio2_003368
113. Kbio2_005132
114. Kbio2_005936
115. Kbio2_007700
116. Kbio3_001595
117. Kbio3_003042
118. Chebi: 28088
119. Cmap_000086
120. Bio1_000445
121. Bio1_000934
122. Bio1_001423
123. Hms2271k09
124. Hms3261h21
125. Hms3267k14
126. Hms3412i13
127. Hms3428m01
128. Hms3649b22
129. Hms3654d17
130. Hms3676i13
131. Hms3742i07
132. Act05962
133. Albb-015886
134. Amy25676
135. Bcp07581
136. Tox21_110161
137. Tox21_201428
138. Tox21_300585
139. Tox21_500520
140. Ac-472
141. Bbl010484
142. Ccg-38551
143. Lmpk12050218
144. S1342
145. Stk801619
146. Who 11073
147. Zinc18825330
148. Akos001590147
149. Tox21_110161_1
150. Cs-1534
151. Db01645
152. Ks-5128
153. Lp00520
154. Sb17235
155. Sdccgsbi-0050503.p003
156. Smp1_000133
157. Genistein; 4',5,7-trihydroxyisoflavone
158. Ncgc00015479-01
159. Ncgc00015479-02
160. Ncgc00015479-04
161. Ncgc00015479-05
162. Ncgc00015479-06
163. Ncgc00015479-07
164. Ncgc00015479-08
165. Ncgc00015479-10
166. Ncgc00015479-11
167. Ncgc00015479-12
168. Ncgc00015479-13
169. Ncgc00015479-14
170. Ncgc00015479-15
171. Ncgc00015479-16
172. Ncgc00015479-17
173. Ncgc00015479-18
174. Ncgc00015479-19
175. Ncgc00015479-20
176. Ncgc00015479-38
177. Ncgc00025005-01
178. Ncgc00025005-02
179. Ncgc00025005-03
180. Ncgc00025005-04
181. Ncgc00025005-05
182. Ncgc00025005-06
183. Ncgc00025005-07
184. Ncgc00169711-01
185. Ncgc00169711-02
186. Ncgc00254275-01
187. Ncgc00258979-01
188. Ncgc00261205-01
189. 690224-00-1
190. Hy-14596
191. Nci60_003369
192. Smr000112580
193. Sy050124
194. Eu-0100520
195. Ft-0603395
196. Ft-0668961
197. Ft-0668962
198. G0272
199. N1861
200. Sw203763-2
201. 46g720
202. C06563
203. D11680
204. G-2535
205. Genistein, Synthetic, >=98% (hplc), Powder
206. K00046
207. Us8552057, 2
208. Ab00052696_09
209. Ab00052696_12
210. 5,7-dihydroxy-3-(4-hydroxyphenyl)-chromen-4-one
211. A826657
212. Q415957
213. Genistein (constituent Of Red Clover) [dsc]
214. Genistein, Primary Pharmaceutical Reference Standard
215. Q-100484
216. Sr-01000075498-1
217. Sr-01000075498-3
218. Sr-01000075498-6
219. 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one
220. Brd-k43797669-001-02-3
221. Brd-k43797669-001-03-1
222. Brd-k43797669-001-10-6
223. Genistein, From Glycine Max (soybean), ~98% (hplc)
224. Sr-01000075498-10
225. 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one
226. F0001-2388
227. Genistein (constituent Of Soy Isoflavones) [dsc]
228. 4h-1-benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-
229. Genistein, United States Pharmacopeia (usp) Reference Standard
230. Genistein, Pharmaceutical Secondary Standard; Certified Reference Material
231. 5,7-dihydroxy-3-(4-hydroxyphenyl)-4h-1-benzopyran-4-one; 4',5,7-trihydroxyisoflavone; Prunetol; Genisteol
| Molecular Weight | 270.24 g/mol |
|---|---|
| Molecular Formula | C15H10O5 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 270.05282342 g/mol |
| Monoisotopic Mass | 270.05282342 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPTL/ Genistein is speculated to provide beneficial effects on cardiovascular and bone health and to alleviate menopausal symptoms; studies examining such endpoints have been limited in number, provided inconsistent findings, or evaluated soy product consumption instead of exposure to genistein alone.
National Toxicology Program, Center For the Evaluation of Risks To Human Reproduction; NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Genistein; (NTP-CERHR-GENISTEIN-06) April 2006. Available from, as of August 14, 2007: https://cerhr.niehs.nih.gov/chemicals/genistein-soy/genistein/Genistein_Report_final.pdf
/EXPTL/ Interest in ... /genistein/ is concentrated in particular on its therapeutic role in menopause. This paper is a review of the main studies published to date on the efficacy of phytoestrogens in reducing the symptoms of menopause. A diet rich in isoflavones is associated with a reduced incidence of vasomotor episodes; the average supplement of genistein is approximately 50 mg/day. After supplementing the diet with phytoestrogens, studies show a reduction in total cholesterol and LDL fraction. This is accompanied by an increase in BMD (Bone mineral density) after taking 90 mg of isoflavones for 6 months. Isoflavones may reduce the risk of developing breast cancer. The data examined confirm the excellent clinical efficacy of supplementing the diet with soy extracts, particularly genistein which is indicated to alleviate both the short-term symptoms of menopause and the long-term effects, although the latter finding requires further subsantiation.
PMID:11828270 Arena S et al; Minerva Ginecol 54 (1): 53-7 (2002)
/EXPTL/ ... /The authors/ evaluated and compared the effects of the phytoestrogen genistein, estrogen-progestogen therapy (EPT), and placebo on hot flushes and endometrial thickness in postmenopausal women. ... Ninety healthy, postmenopausal women, 47 to 57 years of age, were randomly assigned to receive for 1 year continuous EPT (n = 30; 1 mg 17beta-estradiol combined with 0.5 mg norethisterone acetate), the phytoestrogen genistein (n = 30; 54 mg/day), or placebo (n = 30). Endometrial safety was evaluated by intravaginal ultrasounds at baseline, 6 and 12 months. ... By comparison with placebo, daily flushes reduced significantly by a mean of 22% (95% CI: -38 to -6.2; P < 0.01) after 3 months, by a mean of 29% (95% CI: -45 to -13; P < 0.001) after 6 months, and by a mean of 24% (95% CI: -43 to -5; P < 0.01) after 12 months of genistein treatment. Flush score decreased by a mean of 53% (95% CI: -79 to -26; P < 0.001) after 3 months, by a mean of 56% (95% CI: -83 to -28; P < 0.001) after 6 months, and by a mean of 54% (95% CI: -74 to -33; P < 0.001) after 12 months of EPT, as compared with placebo. No side effect was observed on the uterus of the participants. ... The present study confirms that genistein might have positive effects on hot flushes without a negative impact on endometrial thickness and suggests a future role of this phytoestrogen as a strategically therapeutic alternative in the management of postmenopausal symptoms.
PMID:15243277 Crisafulli A et al; Menopause 11 (4): 400-4 (2004)
/EXPTL/ There is a growing body of in vitro and animal studies suggesting that genistein may be helpful in preventing and treating some cancers, principally breast and prostate cancers. The clinical studies that might support or refute claims that genistein has anti-atherogenic properties and that it can safely and effectively be used as natural estrogen-replacement therapy have not been conducted. There are, however, preliminary data suggesting that soy isoflavones, including genistein, may be helpful in some problems associated with menopause, including osteoporosis and hot flashes.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
For more Therapeutic Uses (Complete) data for GENISTEIN (6 total), please visit the HSDB record page.
Genistein/genistin intake has been associated with hypothyroidism in some.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
Women with estrogen receptor-positive tumors should exercise caution in the use of genistein/genistin supplements and should only use them if they are recommended and monitored by a physician.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
Men with prostate cancer should discuss the advisability of the use of genistein/genistin supplements with their physicians before deciding to use them.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
Pregnant women and nursing mothers should avoid the use of genistein/genistin supplements pending long-term safety studies.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
Caution is warranted for postmenopausal women consuming dietary genistein while on tamoxifen therapy for estrogen-responsive breast cancer.
PMID:11980635 Ju YH et al; Cancer Res 62(9):2474-7 (2002)
Currently Genistein is being studied in clinical trials as a treatment for prostate cancer.
Phytoestrogens
Compounds derived from plants, primarily ISOFLAVONES that mimic or modulate endogenous estrogens, usually by binding to ESTROGEN RECEPTORS. (See all compounds classified as Phytoestrogens.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
Anticarcinogenic Agents
Agents that reduce the frequency or rate of spontaneous or induced tumors independently of the mechanism involved. (See all compounds classified as Anticarcinogenic Agents.)
... Genistein is rapidly absorbed in humans following oral intake. Before absorption into the systemic circulation, most genistein is conjugated with glucuronic acid and excreted in the bile to undergo enterohepatic circulation ... . Therefore, genistein bioavailability is very limited. Times to obtain maximum plasma concentrations were reported at 1 to 6 hours for free genistein ... and 3 to 8 hours for total genistein (aglycone + conjugates ...). In one of the studies, the lowest dose used (2 mg/kg bw) was stated to provide more than twice the level of isoflavones ingested in a Japanese daily diet. A study in which menopausal women were given a 50 mg commercial isoflavone extract incorporated into fruit juice, chocolate, or a cookie showed no significant effect of the food matrix on genistein absorption or urinary excretion parameters. In a study in which 8 women were dosed with 0.4 or 0.8 mg/kg bw 13C-labeled genistein, the area under the curve (AUC) at the higher dose was less than double the AUC at the lower dose, suggesting a decrease in fractional absorption with increasing dose.
National Toxicology Program, Center For the Evaluation of Risks To Human Reproduction; NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Genistein; (NTP-CERHR-GENISTEIN-06) April 2006. Available from, as of August 14, 2007: https://cerhr.niehs.nih.gov/chemicals/genistein-soy/genistein/Genistein_Report_final.pdf
There is considerable individual variation in the absorption and metabolism of ingested genistin and genistein. There are some data suggesting that genistein may be more bioavailable than genistin. However, other data suggest that the extent of absorption of genistein is similar for the aglycone and the glucoside forms. There are little data available on the tissue distribution of genistein.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.180 (2001)
A recently completed study has also shown inter-individual variation in the urinary excretion of isoflavones and their metabolites following soy challenge in adults. In this study, 76 volunteers were fed either a high (104+/-24 mg total isoflavones/day) or low (0.5+/-0.5 mg total isoflavones/day) soya diet for 10 weeks. Volunteers on the high soya diet showed extensive urinary excretion of daidzein, genistein and their metabolites. Of the volunteers on the high soya diet 34% were identified as good equol excretors ( 1000 nmol/24 hours). Comparative analysis of the fecal flora between equol and non-equol producers was investigated, however, the microflora (bacteria) responsible for equol production could not be isolated and therefore, were not be identified
Hughs I, Woods HF; Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment Phytoestrogens and Health. UK Food Standards Agency, 444 p. (May 2003). Available from, as of August 14, 2007: https://www.food.gov.uk/multimedia/pdfs/phytoreport0503
The pharmacokinetics of isoflavones in 10 healthy women were determined from serum appearance/disappearance concentration profiles and urinary excretions after single-bolus ingestion of 10, 20 or 40 g of soy nuts delivering increasing amounts of the conjugated forms of daidzein (6.6, 13.2 and 26.4 mg) and genistein (9.8, 19.6 and 39.2 mg). Peak serum daidzein and genistein concentrations were attained after 4-8 hr, and elimination half-lives were 8.0 and 10.1 hr, respectively. There were no differences in the pharmacokinetics of daidzein and genistein between pre- and postmenopausal women, indicating absorption and disposition of isoflavones to be independent of age or menopausal status. A curvilinear relationship was observed between the bioavailability of daidzein and genistein, apparent from the area under the curve to infinity (AUC(inf)) of the serum concentration-time profiles and the amount of isoflavones ingested. The mean fraction of the isoflavones excreted in urine decreased with increasing intake when expressed as a percentage of the administered dose (63.2 + or - 8.0, 54.4 + or - 8.1 and 44.0 + or - 4.3%, respectively, for daidzein, and correspondingly, 25.2 + or - 5.3, 13.4 + or - 2.1 and 15.8 + or - 2.7% for genistein), underscoring the trend toward nonlinear pharmacokinetics. Equol was identified as a metabolite in 30% of women; it was present consistently in urine and blood from the same subjects. Its delayed appearance was consistent with colonic synthesis. On the basis of the pharmacokinetics, optimum steady-state serum isoflavone concentrations would be expected from modest intakes of soy foods consumed regularly throughout the day rather than from a single highly enriched product.
PMID:12672914 Setchell KD et al; J Nutr 133 (4): 1027-35 (2003)
For more Absorption, Distribution and Excretion (Complete) data for GENISTEIN (15 total), please visit the HSDB record page.
Toxicokinetic and metabolism data in humans and experimental animals indicate that genistein is absorbed into the systemic circulation of infants and adults. Genistein ... circulates as its glucuronide conjugate, and a much smaller percentage circulates as the aglycone. Genistein can be glucuronidated in the intestine or liver, but the intestine appears to play the major role in glucuronidation. Genistein glucuronides undergo enterhepatic cycling, and in the process can be deconjugated by intestinal bacteria. The role of gut bacteria in the metabolism of genistein has been clearly established. Genistein can be metabolized through a pathway that ultimately leads to the formation of 6'-hydroxy-O-demethylangolensin. Once absorbed, genistein glucuronide, and to a smaller extent genistien aglycone, are widely distributed to organ systems and the conceptus. The majority of a genistein dose is excreted in urine within 24 hours.
National Toxicology Program, Center For the Evaluation of Risks To Human Reproduction; NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Genistein; (NTP-CERHR-GENISTEIN-06) April 2006. Available from, as of August 14, 2007: https://cerhr.niehs.nih.gov/chemicals/genistein-soy/genistein/Genistein_Report_final.pdf
Prior to entering the systemic circulation, most genistein is conjugated with glucuronic acid by uridine diphosphate (UDP)-glucuronosyltransferase (UDPGT); a much smaller amount is conjugated to sulfate by sulfotransferase enzymes. Conjugation of genistein occurs in the intestine, although it also has been reported to occur in liver. One study demonstrated that the ability to catalyze glucuronidation of genistein was greatest with microsomes from kidney > colon > liver. UDPGT isoenzymes including 1A1, 1A4, 1A6, 1A7, 1A9, and 1A10 were observed to catalyze the glucuronidation of genistein. The UGT 1A10 isoform, which is present in colon, gastric, and biliary epithelium but not in liver, was observed to have the highest activity and specificity for genistein. Based on those observations, the study authors concluded that the intestine plays a major role in the glucuronidation of genistein. The glucuronide and sulfate conjugates can enter the systemic circulation, and the majority of isoflavone compounds in the circulation are present in conjugated form. In studies where humans were exposed to genistein alone or in combination with other isoflavone aglycones (calculated as genistein doses of 1-16 mg/kg bw), most of the genistein was present in plasma in conjugated form; free genistein represented 1-3% of total plasma genistein levels. The conjugated isoflavones undergo enterohepatic circulation, and upon return to the intestine, they are deconjugated by bacteria possessing beta-glucuronidase or arylsulfatase activity. The metabolites may be reabsorbed or further metabolized by gut microflora. One review reported that about 10% of isoflavonoids are circulated in plasma unconjugated.
National Toxicology Program, Center For the Evaluation of Risks To Human Reproduction; NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Genistein; (NTP-CERHR-GENISTEIN-06) April 2006. Available from, as of August 14, 2007: https://cerhr.niehs.nih.gov/chemicals/genistein-soy/genistein/Genistein_Report_final.pdf
Biotransformations by gut microflora play a pivotal role in determining the biological activity of isoflavones that occur in soya-based foods predominantly as betaglycosyl conjugates. Microflora prepared from rat caeca and human feces were used to investigate the metabolic fate of genistein beta-glycosides extracted from soya flour. The end-products of such metabolism were determined by parallel incubations of microflora with [2',3,5',6'-3H] and [4-14C]-labelled genistein. ... Quantitative analysis by LC-MS/IS indicated very rapid and complete degradation of genistin, which was associated with a transient increase in genistein. Qualitative studies indicated that the malonyl and acetyl glycosides of genistein were also degraded by the microflora. ... Incubation of caecal and fecal microflora with (3)H and (14)C genistein yielded similar radiolabelled metabolites, which were identified by radio-LC-MS(n) as the intermediates dihydrogenistein and 6'-hydroxy-O-desmethylangolensin and end-product 4-hydroxyphenyl-2-propionic acid. This profile of genistein metabolites indicated selective hydrolysis of 6'-hydroxy-O-desmethylangolensin between carbon atoms 1' and 1 to yield the end-products 4-hydroxyphenyl-2-propionic acid and 1,3,5-trihydroxybenzene. ... The biological significance of the products of genistein metabolism warrant further investigation since they may play an important role in mediating the beneficial antioxidant health effects associated with the consumption of isoflavones in food.
PMID:11820509 Coldham NG et al; Xenobiotica 32 (1): 45-62 (2002)
Biotransformation of the phytoestrogen (14-C)genistein was investigated in male and female rats by application of narrow-bore radio-HPLC-MSn (LCQ, Finnigan) to determine intermediates in metabolism. Urine contained five metabolites, Gm1-Gm5, 24 hr after dosing by gavage with [14C]genistein (4 mg kg(-1)). Structural analysis following ESI revealed molecular ions (M+H)+ of m/z 447, 449, 273, and 271 for metabolites Gm2, Gm3, Gm5 and genistein, respectively and an [M-H]- of m/z 349 for Gm4. Metabolite structure was deduced by evaluation of product ion spectra derived from unlabelled and (14)C-labelled ions and sensitivity to treatment with beta-glucuronidase. These studies indicated identity of metabolites with genistein glucuronide (Gm2), dihydrogenistein glucuronide (Gm3), genistein sulphate (Gm4) and dihydrogenistein (Gm5). Detection of the beta-glucuronidase resistant major metabolite Gm1 by ESI was poor and so was analysed by negative ion APCI; this revealed a deprotonated molecular ion of m/z 165 which had chromatographic and mass spectral properties consistent with authentic 4-hydroxyphenyl-2-propionic acid, a novel metabolite of genistein. In vitro metabolism studies with anaerobic caecal cultures derived from male and female rats revealed metabolism of genistein to Gm1 via Gm5 and an additional metabolite (Gm6) which was identified from product ion spectra as 6'-hydroxy-O-desmethylangolensin. Biotransformation of genistein by both isolated hepatocytes and precision-cut liver slices was limited to glucuronidation of parent compound. Commonality of genistein metabolites found in rats with those reported in man suggest similar pathways of biotransformation, primarily involving gut micro-flora.
PMID:10622405 Coldham NG et al; J Steroid Biochem Mol Biol 70 (4-6): 169-84 (1999)
For more Metabolism/Metabolites (Complete) data for GENISTEIN (7 total), please visit the HSDB record page.
Genistein has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid, Dihydrogenistein, and Orobol.
Genistein is a known human metabolite of biochanin a.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... Thirty healthy men ingested a single dose of 1 of 2 isoflavone preparations purified from soy. The delivered doses of genistein (1, 2, 4, 8, or 16 mg/kg body wt) were higher than those previously administered to humans. Formulation A was composed of 90 +/- 5% genistein, 10% daidzein, and 1% glycitein. Formulation B was composed of 43% genistein, 21% daidzein, and 2% glycitein. ... The mean elimination half-lives with both formulations were 3.2 hr for free genistein and 4.2 hr for free daidzein. The mean pseudo half-lives were 9.2 hr for total genistein and 8.2 hr for total daidzein.
PMID:11756070 Busby MG et al; Am J Clin Nutr 75 (1): 126-36 (2002)
The pharmacokinetics of isoflavones in 10 healthy women were determined from serum appearance/disappearance concentration profiles and urinary excretions after single-bolus ingestion of 10, 20 or 40 g of soy nuts delivering increasing amounts of the conjugated forms of daidzein (6.6, 13.2 and 26.4 mg) and genistein (9.8, 19.6 and 39.2 mg). Peak serum daidzein and genistein concentrations were attained after 4-8 hr, and elimination half-lives were 8.0 and 10.1 hr, respectively.
PMID:12672914 Setchell KD et al; J Nutr 133 (4): 1027-35 (2003)
Mass balance, plasma pharmacokinetics, tissue distribution, and metabolism of (14-C)genistein were investigated in male and female rats (n = 5) following an oral dose of (14-C)genistein (4 mg/kg) to determine potential sites and mechanisms of biological action. Mean total excretion of radioactivity in urine and feces for both sexes was 66 and 33% of the dose respectively at 166 hr after administration. Mean and maximal concentrations of radioactivity in plasma were significantly (P < 0.02) higher in male than female rats, with half-lives of 12.4 and 8.5 hr, respectively.
PMID:10764634 Coldham NG et al; Toxicol Appl Pharmacol 164 (2): 206-15 (2000)
Genistein may inhibit cancer cell growth by blocking enzymes required for cell growth. Genistein may decrease cardiovascular risk in postmenopausal women by interacting with the nuclear estrogen receptors to alter the transcription of cell specific genes. In randomized clinical trials, genistein was seen to increase the ratio of nitric oxide to endothelin and improved flow-mediated endothelium dependent vasodilation in healthy postmenopausal women. In addition, genistein may have beneficial effects on glucose metabolism by inhibiting islet tyrosine kinase activity as well as insulin release dependent on glucose and sulfonylurea.
Several mechanisms have been proposed for genistein's putative anticarcinogenic activity. These include upregulation of apoptosis, inhibition of angiogenesis, inhibition of DNA topoisomerase II and inhibition of protein tyrosine kinases. Genistein's weak estrogenic activity has been suggested as another mechanism for genistein's putative anti-prostate cancer activity. In addition to the above mechanisms, other mechanisms of genistein's putative anti-prostate cancer activity include inhibition of nuclear factor (NF)-Kappa B in prostate cancer cells, downregulation of TGF (transforming growth factor)-beta and inhibition of EGF (epidermal growth factor)-stimulated growth. Genistein's anti-estrogenic action may be another possible mechanism to explain its putative anti-breast cancer activity. In the final analysis, the mechanism of genistein's putative anticarcinogenic activity is unclear.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.179 (2001)
A number of studies have shown that genistein can interact with topoisomerase II in vitro. ... Genistein (3.7 M) stabilizes the topoisomerase II-DNA complex. ... stimulating topoisomerase II dependent DNA cleavage (in the ranges 80-370 M and 12.5-250 M, respectively). ... Genistein 50 M) inhibited unwinding of DNA by topoisomerase II in primary hematopoietic cells and stimulated strand breakage. ...The effect of genistein on the DNA cleavage and ATP hydrolysis steps of the topoisomerase II catalysed reactions /was investigated and/ genistein inhibited enzyme-catalysed ATP hydrolysis with an IC50 of 7 M. However, genistein did not stimulate DNA cleavage. Further studies ... showed that genistein (50 M) and daidzein (100 M) induced cleavage of the MLL gene in primary hematopoietic cells. Further in vitro experiments suggested that inhibition of topoisomerase II catalysed re-ligation of DNA strand breaks as the mechanism of action.
Hughs I, Woods HF; Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment Phytoestrogens and Health. UK Food Standards Agency, 444 p. (May 2003). Available from, as of August 14, 2007: https://www.food.gov.uk/multimedia/pdfs/phytoreport0503
Genistein has been found to have a number of antioxidant activities. It is a scavenger of reactive oxygen species and inhibits lipid peroxidation. It also inhibits superoxide anion generation by the enzyme xanthine oxidase. In addition, genistein, in animal experiments, has been found to increase the activities of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, catalase and glutathione reductase.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.179 (2001)
Genistein has weak estrogenic activity as measured in in vivo and in vitro assays. In vivo, its estrogenic activity is one-third that of glycitein and four times greater than that of daidzein.
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ p.179 (2001)
For more Mechanism of Action (Complete) data for GENISTEIN (9 total), please visit the HSDB record page.