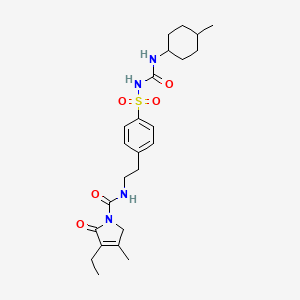

1. 1-(4-(2-(3-ethyl-4-methyl-2-oxo-3-pyrrolinecarboxamido)ethyl)phenylsulfonyl)-3-(4-methylcyclohexyl)urea
2. Amarel
3. Amaryl
4. Glymepiride
5. Hoe 490
6. Hoe-490
7. Roname
1. 93479-97-1
2. Amaryl
3. Glimepirid
4. Amarel
5. Glimepirida
6. Glimepiridum
7. Hoe-490
8. Glimepride
9. Hoe 490
10. Cis-glimepiride
11. 684286-46-2
12. Glimepiride, Cis-
13. Glimperide
14. Glimepiride Impurity A
15. 4-ethyl-3-methyl-n-[2-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-5-oxo-2h-pyrrole-1-carboxamide
16. Chebi:5383
17. 1-((p-(2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl)phenyl)sulfonyl)-3-(trans-4-methylcyclohexyl)urea
18. Endial
19. Nsc-759809
20. 24t6xir2mz
21. 6ky687524k
22. 261361-60-8
23. Ncgc00016960-03
24. Glimepiridum [latin]
25. Roname
26. Glimepirida [spanish]
27. Cas-93479-97-1
28. Glista Od
29. Dsstox_cid_20675
30. Dsstox_rid_79534
31. Dsstox_gsid_40675
32. 1h-pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-n-(2-(4-(((((4-methylcyclohexyl)amino)carbonyl)amino)sulfonyl)phenyl)ethyl)-2-oxo-, Trans-
33. 3-ethyl-4-methyl-n-[2-(4-{[(trans-4-methylcyclohexyl)carbamoyl]sulfamoyl}phenyl)ethyl]-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
34. Glymepirid
35. Glorion
36. Glemax
37. Glimer
38. Solosa
39. 3-ethyl-4-methyl-n-[2-(4-{[(cis-4-methylcyclohexyl)carbamoyl]sulfamoyl}phenyl)ethyl]-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
40. Smr000466368
41. Amaryl (tn)
42. Ccris 7083
43. Sr-05000001508
44. Unii-24t6xir2mz
45. Brn 5365754
46. Gimepiride
47. Vitamine
48. Niddaryl
49. Sugral
50. Hoe490
51. Unii-6ky687524k
52. Glimepiride,(s)
53. 1-[[4-[2-[[(3-ethyl-4-methyl-2-oxo-2,3-dihydro-1h-pyrrol-1-yl)carbonyl]amino]ethyl]phenyl]sulphonyl]-3-(cis-4-methylcyclohexyl)urea (cis-glimepiride)
54. 3-ethyl-4-methyl-n-[2-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-2-oxo-5h-pyrrole-1-carboxamide
55. Glimepiride [usan:usp:inn:ban]
56. Amaryl, Glista Od
57. Glimepiride- Bio-x
58. Mfcd00878417
59. Cpd000466368
60. Glimepiride [mi]
61. Prestwick0_000651
62. Prestwick1_000651
63. Prestwick2_000651
64. Prestwick3_000651
65. Glimepiride [inn]
66. Glimepiride [jan]
67. Glimepiride [usan]
68. Glimepiride [vandf]
69. Chembl1481
70. Glimepiride [mart.]
71. Oprea1_382896
72. Schembl16084
73. Schembl16086
74. Bspbio_000681
75. Glimepiride [usp-rs]
76. Glimepiride [who-dd]
77. Glimepiride Cis-isomer
78. Mls000759495
79. Mls001076674
80. Mls001401419
81. Mls003915622
82. Mls006011260
83. Spbio_002602
84. Glimepiride [ema Epar]
85. Bpbio1_000751
86. Chembl149223
87. Gtpl6820
88. Schembl8738802
89. Glimepiride (jp17/usp/inn)
90. Dtxsid5040675
91. Schembl14371714
92. Schembl14965363
93. Chebi:92609
94. Dtxsid20861130
95. Glimepiride [orange Book]
96. Glimepiride For System Suitability
97. Glimepiride [ep Monograph]
98. Glimepiride [usp Impurity]
99. Hms1570c03
100. Hms2052l03
101. Hms2090k18
102. Hms2097c03
103. Hms2235l07
104. Hms3269a09
105. Hms3372o07
106. Hms3394l03
107. Hms3413k06
108. Hms3654f17
109. Hms3677k06
110. Hms3714c03
111. Pharmakon1600-01504915
112. Zinc537791
113. Glimepiride [usp Monograph]
114. Bcp05331
115. Duetact Component Glimepiride
116. Hy-b0104
117. Tox21_110713
118. Ac-476
119. Bdbm50237590
120. Nsc759809
121. Nsc813217
122. S1344
123. Stl451059
124. Stl453194
125. Glimepiride Related Compound A
126. Akos015894919
127. Akos015969663
128. Glimepiride, >=98% (hplc), Solid
129. Tox21_110713_1
130. Zinc100001976
131. Zinc100070954
132. Ab07644
133. Bcp9000728
134. Ccg-101156
135. Cs-1844
136. Db00222
137. Glimepiride Component Of Duetact
138. Ks-5238
139. Nc00406
140. Nsc 759809
141. Nsc-813217
142. Ncgc00016960-01
143. Ncgc00016960-02
144. Ncgc00016960-04
145. Ncgc00016960-05
146. Ncgc00016960-07
147. Ncgc00161404-01
148. Ncgc00161404-02
149. Ncgc00181757-01
150. Ncgc00371061-02
151. Ncgc00371061-06
152. 1-{[4-(2-{[(3-ethyl-4-methyl-2-oxo-2,5-dihydro-1h-pyrrol-1-yl)carbonyl]amino}ethyl)phenyl]sulfonyl}-3-(trans-4-methylcyclohexyl)urea
153. 1h-pyrrole-1-carboxamide, 2,5-dihydro-3-ethyl-4-methyl-n-(2-(4-(((((4-methylcyclohexyl)amino)carbonyl)amino)sulfonyl)phenyl)ethyl)-2-oxo-, Trans-
154. 3-ethyl-4-methyl-n-(4-(n-((1r,4r)-4-methylcyclohexylcarbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro
155. Bg164507
156. Smr001550123
157. Glimepiride 100 Microg/ml In Acetonitrile
158. Ab00513874
159. Cs-0165191
160. Ft-0626713
161. Ft-0668978
162. G0395
163. Glimepiride Impurity A [ep Impurity]
164. Sw196369-4
165. C07669
166. D00593
167. Glimepiride Related Compound A [usp-rs]
168. Ab00513874-06
169. Ab00513874-08
170. Ab00513874-09
171. Ab00513874_10
172. Ab00513874_11
173. 479g971
174. A844609
175. A899888
176. Q425027
177. Q-201158
178. Sr-05000001508-1
179. Sr-05000001508-2
180. Sr-05000001508-3
181. Brd-k34776109-001-03-4
182. Brd-k42693031-001-01-8
183. Glimepiride Related Compound A [usp Impurity]
184. Q27253874
185. Glimepiride, British Pharmacopoeia (bp) Reference Standard
186. Glimepiride, European Pharmacopoeia (ep) Reference Standard
187. 3-ethyl-4-methyl-n-(4-(n-((1r,4r)-4-methylcyclohexylcarbamoyl)
188. Glimepiride, United States Pharmacopeia (usp) Reference Standard
189. Sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
190. Glimepiride For System Suitability, European Pharmacopoeia (ep) Reference Standard
191. 1-((4-(2-(((3-ethyl-4-methyl-2-oxo-2,3-dihydro-1h-pyrrol-1-yl)carbonyl)amino)ethyl)phenyl)sulfonyl)-3-(cis-4-methylcyclohexyl)urea
192. 1h-pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-n-(2-(4-(((((cis-4-methylcyclohexyl)amino)carbonyl)amino)sulfonyl)phenyl)ethyl)-2-oxo-
193. 1h-pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[(trans- 4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-
194. 3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[(4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1h-pyrrole-1-carboxamide
195. 3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[(trans-4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1h-pyrrole-1-carboxamide
196. 3-ethyl-4-methyl-2-oxo-n-(2-{4-[({[(1r,4r)-4-methylcyclohexyl]carbamoyl}amino)sulfonyl]phenyl}ethyl)-2,5-dihydro-1h-pyrrole-1-carboxamide
197. 3-ethyl-4-methyl-n-(4-(n-(((1r,4r)-4-methylcyclohexyl)carbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
198. 3-ethyl-4-methyl-n-(4-(n-(((1s,4s)-4-methylcyclohexyl)carbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide? (glimepiride Impurity Pound(c)
199. 3-ethyl-4-methyl-n-(4-(n-((1r,4r)-4-methylcyclohexylcarbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
200. 3-ethyl-4-methyl-n-(4-(n-((rel-(1r,4r)-4-methylcyclohexyl)carbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
201. 3-ethyl-4-methyl-n-(4-(n-((trans-4-methylcyclohexyl)carbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
202. 3-ethyl-4-methyl-n-[2-(4-{[(4-methylcyclohexyl)carbamoyl]sulfamoyl}phenyl)ethyl]-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
203. 3-ethyl-4-methyl-n-[2-(4-{[(cis-4-methylcyclohexyl)carbamoyl]sulfamoyl}phenyl)ethyl]-2-oxo-2,5-dihydro-1h-pyrrole-1-car
204. 3-ethyl-4-methyl-n-[2-[4-[[3-(4-methylcyclohexyl)ureido]sulfonyl]phenyl]ethyl]-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
205. 3-ethyl-4-methyl-n-{2-[4-({[(4-methylcyclohexyl)carbamoyl]amino}sulfonyl)phenyl]ethyl}-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamide
206. N'-{[4-(2-{[(3-ethyl-4-methyl-2-oxo-2,5-dihydro-1h-pyrrol-1-yl)carbonyl]amino}ethyl)phenyl]sulfonyl}-n-(4-methylcyclohexyl)carbamimidic Acid
207. N-(4-ethyl-3-methyl-5-oxo-2h-pyrrol-1-yl)-3-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]propanamide;glimepiride
208. N-[4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)-ethyl]-benzenesulfonyl]-n'-4-methylcyclohexylurea
209. Trans-1-(4-(2-(3-ethyl-4-me-2-oxo-2,5-dihydro-1h-pyrrole-1-carboxamido)ethyl)phenylsulfonyl)-3-(4-methylcyclohexyl)urea
210. Trans-3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[(4-methyl Cyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1h-pyrrole-1-carboxamide
211. Trans-3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[(4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1h-pyrrole-1-carboxamide
212. Trans-3-ethyl-2,5-dihydro-4-methyl-n-[2-[4-[[[[trans-4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1h-pyrrole-1-carboxyamide
| Molecular Weight | 490.6 g/mol |
|---|---|
| Molecular Formula | C24H34N4O5S |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 490.22499137 g/mol |
| Monoisotopic Mass | 490.22499137 g/mol |
| Topological Polar Surface Area | 133 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 895 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Amaryl |
| PubMed Health | Glimepiride (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | AMARYL is an oral sulfonylurea that contains the active ingredient glimepiride. Chemically, glimepiride is identified as 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido) ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea (C24H34N4O5S)... |
| Active Ingredient | Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 4 | |
|---|---|
| Drug Name | Glimepiride |
| PubMed Health | Glimepiride (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | Glimepiride Tablets, USP are an oral blood-glucose-lowering drug of the sulfonylurea class. Glimepiride, USP is a white to yellowish-white, crystalline, odorless to practically odorless powder formulated into tablets of 1-mg, 2-mg, and 4-mg strengths... |
| Active Ingredient | Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg; 8mg; 4mg; 6mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Teva; Accord Hlthcare; Aurobindo Pharma; Invagen Pharms; Hikma Pharms; Indoco Remedies; Micro Labs Ltd India; Vintage; Dr Reddys Labs; Carlsbad; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Amaryl |
| PubMed Health | Glimepiride (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | AMARYL is an oral sulfonylurea that contains the active ingredient glimepiride. Chemically, glimepiride is identified as 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido) ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea (C24H34N4O5S)... |
| Active Ingredient | Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 4 of 4 | |
|---|---|
| Drug Name | Glimepiride |
| PubMed Health | Glimepiride (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | Glimepiride Tablets, USP are an oral blood-glucose-lowering drug of the sulfonylurea class. Glimepiride, USP is a white to yellowish-white, crystalline, odorless to practically odorless powder formulated into tablets of 1-mg, 2-mg, and 4-mg strengths... |
| Active Ingredient | Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg; 8mg; 4mg; 6mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Teva; Accord Hlthcare; Aurobindo Pharma; Invagen Pharms; Hikma Pharms; Indoco Remedies; Micro Labs Ltd India; Vintage; Dr Reddys Labs; Carlsbad; Mylan |
Glimepiride is indicated for the management of type 2 diabetes in adults as an adjunct to diet and exercise to improve glycemic control as monotherapy. It may also be indicated for use in combination with metformin or insulin to lower blood glucose in patients with type 2 diabetes whose high blood sugar levels cannot be controlled by diet and exercise in conjunction with an oral hypoglycemic (a drug used to lower blood sugar levels) agent alone.
Glimepiride stimulates the secretion of insulin granules from the pancreatic beta cells and improves the sensitivity of peripheral tissues to insulin to increase peripheral glucose uptake, thus reducing plasma blood glucose levels and glycated hemoglobin (HbA1C) levels. A multi-center, randomized, placebo-controlled clinical trial evaluated the efficacy of glimepiride (18 mg) as monotherapy titrated over 10 weeks compared with placebo in T2DM subjects who were not controlled by diet alone. In this study, there was a reduction in fasting plasma glucose (FPG) by 46 mg/dL, post-prandial glucose (PPG) by 72 mg/dL, and HbA1c by 1.4% more than the placebo. In another randomized study comprising of patients with T2DM receiving either placebo or one of the three doses (1, 4, or 8 mg) of glimepiride during a 14-week study period, all glimepiride regimens significantly reduced FPG, PPG, and HbA1c values (P < 0.001) compared to placebo by the end of the study period. The 4- and 8-mg doses of glimepiride were more effective than the 1-mg dose; however, the 4-mg dose provided a nearly maximal antihyperglycemic effect.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
A10BB12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BB - Sulfonylureas
A10BB12 - Glimepiride
Absorption
Glimepiride is completely absorbed after oral administration within 1 hour of administration with a linear pharmacokinetics profile. Following administration of a single oral dose of glimepiride in healthy subjects and with multiple oral doses with type 2 diabetes, the peak plasma concentrations (Cmax) were reached after 2 to 3 hours post-dose. Accumulation does not occur after multiple doses. When glimepiride was given with meals, the time to reach Cmax was increased by 12% while the mean and AUC (area under the curve) were decreased by 8 to 9%, respectively. In a pharmacokinetic study of Japanese patients with T2DM, Cmax value in once-daily dose was higher than those in twice-daily doses. The absolute bioavailability of glimepiride is reported to be complete following oral administration.
Route of Elimination
Following oral administration of glimepiride in healthy male subjects, approximately 60% of the total radioactivity was recovered in the urine in 7 days, with M1 and M2 accounting for 80-90% of the total radioactivity recovered in the urine. The ratio of M1 to M2 was approximately 3:2 in two subjects and 4:1 in one subject. Approximately 40% of the total radioactivity was recovered in feces where M1 and M2 accounted for about 70% of the radioactivity and a ratio of M1 to M2 being 1:3. No parent drug was recovered from urine or feces.
Volume of Distribution
Following intravenous dosing in healthy subjects, the volume of distribution was 8.8 L (113 mL/kg).
Clearance
A single-dose, crossover, dose-proportionality (1, 2, 4, and 8 mg) study in normal subjects and from a single- and multiple-dose, parallel, dose proportionality (4 and 8 mg) study in patients with type 2 diabetes (T2D) were performed. In these studies, the total body clearance was 52.1 +/- 16.0 mL/min, 48.5 +/- 29.3 mL/min in patients with T2D given a single oral dose, and 52.7 +/- 40.3 mL/min in patients with T2D given multiple oral doses. Following intravenous dosing in healthy subjects, the total body clearance was 47.8 mL/min.
Glimepiride is reported to undergo hepatic metabolism. Following either an intravenous or oral dose, glimepiride undergoes oxidative biotransformation mediated by CYP2C9 enzyme to form a major metabolite, cyclohexyl hydroxymethyl derivative (M1), that is pharmacologically active. M1 can be further metabolized to the inactive metabolite carboxyl derivative (M2) by one or several cytosolic enzymes. M1 retained approximately one third of the pharmacologic activity of its parent in an animal model, with a half-life of 3-6 hours. However, whether the glucose-lowering effect of M1 is clinically significant is not clear.
Glimepiride has known human metabolites that include Cyclohexylhydroymethylglimepiride.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life of glimepiride is approximately 5 to 8 hours, which can increase up to 9 hours following multiple doses.
ATP-sensitive potassium channels on pancreatic beta cells that are gated by intracellular ATP and ADP. The hetero-octomeric complex of the channel is composed of four pore-forming Kir6.2 subunits and four regulatory sulfonylurea receptor (SUR) subunits. Alternative splicing allows the formation of channels composed of varying subunit isoforms expressed at different concentrations in different tissues. In pancreatic beta cells, ATP-sensitive potassium channels play a role as essential metabolic sensors and regulators that couple membrane excitability with glucose-stimulated insulin secretion (GSIS). When there is a decrease in the ATP:ADP ratio, the channels are activated and open, leading to K+ efflux from the cell, membrane hyperpolarization, and suppression of insulin secretion. In contrast, increased uptake of glucose into the cell leads to elevated intracellular ATP:ADP ratio, leading to the closure of channels and membrane depolarization. Depolarization leads to activation and opening of the voltage-dependent Ca2+ channels and consequently an influx of calcium ions into the cell. Elevated intracellular calcium levels causes the contraction of the filaments of actomyosin responsible for the exocytosis of insulin granules stored in vesicles. Glimepiride blocks the ATP-sensitive potassium channel by binding non-specifically to the B sites of both sulfonylurea receptor-1 (SUR1) and sulfonylurea receptor-2A (SUR2A) subunits as well as the A site of SUR1 subunit of the channel to promote insulin secretion from the beta cell.