
1. Big Plasma Glucagon
2. Glucagon-like-immunoreactivity
3. Gut Glucagon-like Immunoreactants
1. Glucagonum
2. Glucagone
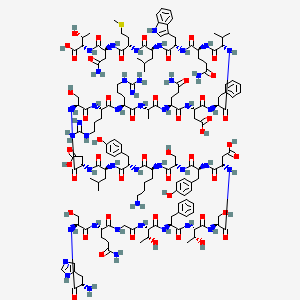
3. His-ser-glu(nh2)-gly-thr-phe-thr-ser-asp-tyr-ser-lys-tyr-leu-asp-ser-arg-arg-ala-glu(nh2)-asp-phe-val-glu(nh2)-trp-leu-met-asp(nh2)-thr
4. Glucagon-like-immunoreactivity
5. Glucaton
6. Bovine Glucagon
7. Glukagon Novo
8. Glucagon, Pig
9. Glucagon (dog)
10. Glucagon (pig)
11. Glucagon (ox)
12. Big Plasma Glucagon
13. Glucagone [dcit]
14. Glucagonum [inn-latin]
15. Glucagon (xenopus Laevis)
16. Glucagon (saimiri Sciureus)
17. Unii-76la80ig2g
18. 76la80ig2g
19. Glucagon (mesocricetus Auratus)
20. Glucagon, Porcine, For Bioassay
21. Glucagon [usp:inn:ban:jan]
22. Schembl15268863
23. Gut Glucagon-like Immunoreactants
24. Hsdb 3337
25. Dtxsid101016809
26. Glucagon, Porcine, For Immunoassay
27. Hsqgtftsdyskyldsrraqdfvqwlmnt
28. Einecs 232-708-2
29. Ncgc00167140-01
30. Glucagon, Acetate Salt, >=97.0% (hplc)
31. Human Glucagon, European Pharmacopoeia (ep) Reference Standard
32. His-ser-gln-gly-thr-phe-thr-ser-asp-tyr-ser-lys-tyr-leu-asp-ser-arg-arg-ala-gln-asp-phe-val-gln-trp-leu-met-asn-thr
33. L-histidyl-l-seryl-l-glutaminylglycyl-l-threonyl-l-phenylalanyl-l-threonyl-l-seryl-l-alpha-aspartyl-l-tyrosyl-l-seryl-l-lysyl-l-tyrosyl-l-leucyl-l-alpha-aspartyl-l-seryl-l-arginyl-l-arginyl-l-alanyl-l-glutaminyl-l-alpha-aspartyl-l-phenylalanyl-l-valyl-l-glutaminyl-l-tryptophyl-l-leucyl-l-methionyl-l-asparaginyl-l-threonine
34. L-threonine, L-histidyl-l-seryl-l-glutaminylglycyl-l-threonyl-l-phenylalanyl-l-threonyl-l-seryl-l-.alpha.-aspartyl-l-tyrosyl-l-seryl-l-lysyl-l-tyrosyl-l-leucyl-l-.alpha.-aspartyl-l-seryl-l-arginyl-l-argin Yl-l-alanyl-l-glutaminyl-l-.alpha.-aspartyl-l-phenylalanyl-l-valyl-l-glutaminyl-l-tryptophyl-l-leucyl-l-methionyl-l-asparaginyl-
35. L-threonine, L-histidyl-l-seryl-l-glutaminylglycyl-l-threonyl-l-phenylalanyl-l-threonyl-l-seryl-l-alpha-aspartyl-l-tyrosyl-l-seryl-l-lysyl-l-tyrosyl-l-leucyl-l-alpha-aspartyl-l-seryl-l-arginyl-l-arginyl-l-alanyl-l-glytaminyl-l-alpha-aspartyl-l-phenylalanyl-l-valyl-l-glutaminyl-l-tryptophyl-l-leucyl-l-methionyl-l-asparaginyl-
| Molecular Weight | 3482.7 g/mol |
|---|---|
| Molecular Formula | C153H225N43O49S |
| XLogP3 | -16.9 |
| Hydrogen Bond Donor Count | 55 |
| Hydrogen Bond Acceptor Count | 55 |
| Rotatable Bond Count | 115 |
| Exact Mass | 3481.6190567 g/mol |
| Monoisotopic Mass | 3480.6157019 g/mol |
| Topological Polar Surface Area | 1560 Ų |
| Heavy Atom Count | 246 |
| Formal Charge | 0 |
| Complexity | 8160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 31 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Gastrointestinal Agents; Protein Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Glucagon is used in the treatment of lower esophageal obstruction due to foreign bodies, including food boluses. /NOT included in US product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1440
Glucagon may be of use in treating myocardial depression due to calcium channel blocking agents in those patients in whom conventional therapies have been ineffective. /NOT included in US product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1440
Glucagon administered in large intravenous doses is used to treat the cardiotoxic effects, specifically bradycardia and hypotension, in overdoses of beta-adrenergic blocking agents. Glucagon may be used with the proterenol or dobutamine. Supplemental potassium may be necessary for treated patients since glucagon tends to reduce serum potassium. /NOT included in US product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1440
For more Therapeutic Uses (Complete) data for GLUCAGON (19 total), please visit the HSDB record page.
...EFFECTIVE ONLY WHEN ADMIN PARENTERALLY. ITS HYPERGLYCEMIC EFFECT IS...OF RELATIVELY BRIEF DURATION. .../SUPPLEMENTARY CARBOHYDRATES SHOULD BE GIVEN AS SOON AS POSSIBLE AFTER PATIENT RESPONDS/. AN ADDITIONAL SUGAR SOURCE IS ESPECIALLY IMPORTANT IN JUVENILES...
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1045
Since glucagon is a protein, the possibility of hypersensitivity reactions should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 2076
Side/Adverse Effects: Those indicating need for medical attention only if they continue or are bothersome: Nausea or vomiting - incidence is generally dependent upon dose and (with intravenous use) the rate of injection; these effects may be diminished by slower intravenous administration.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1441
Glucagon should not be used to treat birth asphyxia or hypoglycemia in premature infants or in infants who have had intrauterine growth retardation.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1440
Glucagon has been used as an aid in the diagnosis of insulinoma and pheochromocytoma; however, USP advisory panels do not generally recommend this use because of questions about safety.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1440
Glucagon is indicated as a diagnostic aid in radiologic exams to temporarily inhibit the movement of the gastrointestinal tract and to treat severe hypoglycemia.
FDA Label
Baqsimi is indicated for the treatment of severe hypoglycaemia in adults, adolescents, and children aged 4 years and over with diabetes mellitus.
Ogluo is indicated for the treatment of severe hypoglycaemia in adults, adolescents, and children aged 2 years and over with diabetes mellitus.
Treatment of hypoglycaemia
Glucagon is indicated as a diagnostic aid in radiologic exams to temporarily inhibit the movement of the gastrointestinal tract and severe hypoglycemia. Glucagon raises blood sugar through activation of hepatic glucagon receptors, stimulating glycogenolysis and the release of glucose. Glucagon has a short duration of action. Glucagon may cause hyperglycemia in diabetic patients.
H04AA01
H04AA01
H - Systemic hormonal preparations, excl. sex hormones and insulins
H04 - Pancreatic hormones
H04A - Glycogenolytic hormones
H04AA - Glycogenolytic hormones
H04AA01 - Glucagon
Absorption
A 1mg intravenous dose of glucagon reaches a Cmax of 7.9ng/mL with a Tmax of 20 minutes. An intramuscular dose reaches a Cmax of 6.9ng/mL with a Tmax of 13 minutes. A 3mg dose of glucagon nasal powder reaches a Cmax of 6130pg/mL with a Tmax of 15 minutes.
Route of Elimination
Elimination of glucagon is not fully characterized in literature, however the kidney and liver appear to contribute significantly in animal models. The liver and kidney are responsible for approximately 30% of glucagon elimination each.
Volume of Distribution
The volume of distribution of glucagon is 0.25L/kg. The apparent volume of distribution is 885L.
Clearance
A 1mg intravenous dose of glucagon has a clearance of 13.5mL/min/kg.
Because of its polypeptide nature, glucagon is destroyed in the GI tract, and therefore must be administered parenterally.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 2075
Glucagon is a protein and so it is metabolized into smaller polypeptides and amino acids in the liver, kidney, and plasma.
The half life of glucagon is 26 minutes for an intramuscular dose. The half life of glucagon nasal powder is approximately 35 minutes. The half life of glucagon by a subcutaneous auto-injector or pre-filled syringe is 32 minutes.
Glucagon has a plasma half-life of about 3-10 minutes.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 2075
Glucagon binds to the glucagon receptor activating Gs and Gq. This activation activates adenylate cyclase, which increases intracellular cyclic AMP and activates protein kinase A. Activating Gq activates phospholipase C, increases production of inositol 1,4,5-triphosphate, and releases intracellular calcium. Protein kinase A phosphorylates glycogen phosphorylase kinase, which phosphorylates glycogen phosphorylase, which phosphorylates glycogen, causing its breakdown. Glucagon also relaxes smooth muscle of the stomach, duodenum, small bowel, and colon.
Glucagon increases the blood glucose concentration by mobilizing hepatic glycogen and thus is effective only when hepatic glycogen is available. Patients with reduced glycogen stores (eg, starvation, adrenal insufficiency, alcoholic hypoglycemia) cannot respond to glucagon.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1045
Glucagon produces extra hepatic effects that are independent of its hyperglycemic action. Although the exact mechanism(s) of action has not been conclusively determined, glucagon produces relaxation of smooth muscle of the stomach, duodenum, small intestine, and colon. The drug has also been shown to inhibit gastric and pancreatic secretions.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 2075
Promotes hepatic glycogenolysis and gluconeogenesis. Stimulates adenylate cyclase to produce increased cyclic-AMP, which is involved in a series of enzymatic activities. The resultant effects are increased concentrations of plasma glucose, a relaxant effect on smooth musculature, and an inotropic myocardial effect. Hepatic stores of glycogen are necessary for glucagon to elicit an antihypoglycemic effect.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1441