

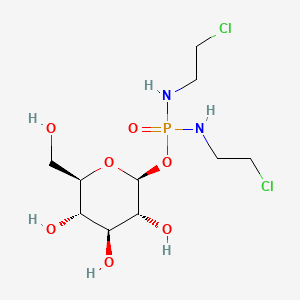

1. Beta-d-glc-ipm
2. Beta-d-glucosyl-ifosfamide Mustard
3. Beta-d-glucosylisophosphoramide Mustard
4. D 19575
5. D-19575
6. Glucosyl-ifosfamide Mustard
1. 132682-98-5
2. D 19575
3. Glufosfamide [inn]
4. Glucosylifosfamide Mustard
5. D-19575
6. 1w5n8szd9a
7. Glucosyl-ifosfamide Mustard
8. Beta-d-glucopyranose, 1-(n,n'-bis(2-chloroethyl)phosphorodiamidate)
9. Glucosylifostamide Mustard
10. Glc-ipm
11. Beta-d-glucopyranose, 1-[n,n'-bis(2-chloroethyl)phosphorodiamidate]
12. N,n'-bis(2-chloroethyl)phosphorodiamidic Acid Beta-d-glucopyranosyl Ester
13. Glufosfamide,b-d-glucopyranose,1-(n,n-bis(2-chloroethyl)phosphorodiamidate)-
14. Hsdb 7024
15. Unii-1w5n8szd9a
16. (2s,3r,4s,5s,6r)-2-bis(2-chloroethylamino)phosphoryloxy-6-(hydroxymethyl)oxane-3,4,5-triol
17. Beta-d-glucopyranose 1-(n,n'-bis(2-chloroethyl)phosphorodiamidate
18. Glufosfamide [mi]
19. Glufosfamide [hsdb]
20. Schembl18241
21. Glufosfamide [mart.]
22. Glufosfamide [who-dd]
23. Chembl2107143
24. Dtxsid001031222
25. Zinc3918137
26. Db06177
27. Hy-16232
28. Cs-0006254
29. 682g985
30. Q15711735
31. .beta.-d-glucopyranose, 1-(n,n'-bis(2-chloroethyl))phosphorodiamidate
| Molecular Weight | 383.16 g/mol |
|---|---|
| Molecular Formula | C10H21Cl2N2O7P |
| XLogP3 | -2 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 382.0463434 g/mol |
| Monoisotopic Mass | 382.0463434 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 369 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... To determine the maximum-tolerated dose (MTD), the principal toxicities, and the pharmacokinetics of 6-hour infusion of glufosfamide (beta-D-glucosylisophosphoramide mustard; D-19575), a novel alkylating agent with the potential to target the glucose transporter system. ... Twenty-one patients (10 women and 11 men; median age, 56 years) with refractory solid tumors were treated with doses ranging from 800 to 6,000 mg/m(2). Glufosfamide was administered every 3 weeks as a two-step (fast/slow) intravenous infusion over a 6-hour period. All patients underwent pharmacokinetic sampling at the first course. ... The MTD was 6,000 mg/m(2). At this dose, two of six patients developed a reversible, dose-limiting renal tubular acidosis and a slight increase in serum creatinine the week after the second and third courses of treatment, respectively, whereas three of six patients experienced short-lived grade 4 neutropenia/leukopenia. Other side effects were generally mild. Pharmacokinetics indicated linearity of area under the time-versus-concentration curve against dose over the dose range studied and a short elimination half-life. There was clear evidence of antitumor activity, with a long-lasting complete response of an advanced pancreatic adenocarcinoma and minor tumor shrinkage of two refractory colon carcinomas and one heavily pretreated breast cancer. ... The principal toxicity of 6-hour infusion of glufosfamide is reversible renal tubular acidosis, the MTD is 6,000 mg/m(2), and the recommended phase II dose is 4, 500 mg/m(2). Close monitoring of serum potassium and creatinine levels is suggested for patients receiving glufosfamide for early detection of possible renal toxicity. Evidence of antitumor activity in resistant carcinomas warrants further clinical exploration of glufosfamide in phase II studies.
PMID:11032596 Briasoulis E, et al; J Clin Oncol 18 (20): 3535-3544 (2000)
Investigated for use/treatment in pancreatic cancer, solid tumors, breast cancer, colorectal cancer, brain cancer, lung cancer, ovarian cancer, and sarcoma.
... /A study was conducted/ to determine the maximum tolerated dose (MTD)... and the pharmacokinetics of 6 hr infusion of glufosfamide ... a novel alkylating agent with the potential to target the glucose transporter system. Twenty-one patients (10 women, 11 men; median age 56 yr) with refractory solid tumors were treated with doses ranging from 800 to 6,000 mg/sq m. /The drug/ was admin very three weeks as a two step (fast/slow) iv infusion over a 6 hr period. All patients went under pharmacokinetic sampling at the first course. The MTD was 6,000 mg/sq m. ... Pharmacokinetics indicated linearity of the area the time-versus-concentration curve against dose over the dose range studied and a short elimination half life. ...
PMID:11032596 Briasoulis E, et al; J Clin Oncol 18 (20): 3535-44 (2000)
Beta-D-glucosyl-ifosfamide mustard (D 19575, glc-IPM, INN = glufosfamide) is a new agent for cancer chemotherapy. Its mode of action, which is only partly understood, was investigated at the DNA level. In the breast carcinoma cell line MCF7 glufosfamide inhibited both the synthesis of DNA and protein in a dose-dependent manner, as shown by the decreased incorporation of (3)H-methyl-thymidine into DNA and (14)C-methionine into protein of these cells. Treatment of MCF7 cells with 50 microM glufosfamide was sufficient to trigger poly(ADP-ribose) polymerase (PARP) activation, as revealed by immunofluorescence analysis. Both CHO-9 cells, which are O6-methylguanine-DNA methyltransferase (MGMT)-deficient, and an isogenic derivative, which has a high level of MGMT, showed the same cytotoxic response to beta-D-glc-IPM, indicating that the O6 position of guanine is not the critical target for cytotoxicity. By contrast, a sharp decrease in survival of cross-link repair deficient CL-V5 B cells was observed already at concentrations of 0.1 mM beta-D-glc-IPM, whereas the wild-type V79 cells showed a 90% reduction in survival only after treatment with 0.5 mM of this compound. The therapeutically inactive beta-L-enantiomer of glufosfamide also showed genotoxic effects in the same assays but at much higher doses. This was probably due to small amounts of ifosfamide mustard formed under the conditions of incubation. The results indicate that the DNA crosslinks are the most critical cytotoxic lesions induced by beta-D-glc-IPM.
PMID:10682676 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363326 Seker H, et al; Br J Cancer 82 (3): 629-634 (2000)