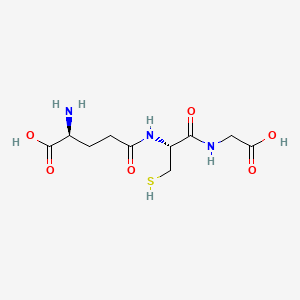

1. Gamma L Glu L Cys Gly
2. Gamma L Glutamyl L Cysteinylglycine
3. Gamma-l-glu-l-cys-gly
4. Gamma-l-glutamyl-l-cysteinylglycine
5. Glutathione, Reduced
6. Reduced Glutathione
1. 70-18-8
2. L-glutathione
3. Glutathion
4. Isethion
5. L-glutathione Reduced
6. Tathion
7. Reduced Glutathione
8. Glutathione-sh
9. Glutinal
10. Tathione
11. Deltathione
12. Neuthion
13. Copren
14. Glutide
15. Triptide
16. Ledac
17. Gsh
18. Glutatione
19. Glutatiol
20. Glutathione Reduced
21. Panaron
22. Glutathione Sh
23. L-glutatione
24. Agifutol S
25. Glutathione (reduced)
26. L-gamma-glutamyl-l-cysteinylglycine
27. Gamma-l-glutamyl-l-cysteinylglycine
28. Glutathione [jan]
29. L-glutathione, Reduced
30. Gamma-l-glutamyl-l-cysteinyl-glycine
31. 5-l-glutamyl-l-cysteinylglycine
32. Glutham
33. Gamma-l-glutamylcysteinylglycine
34. Glutathione Red
35. Aec Glutathione
36. Red. Glutathione
37. Bakezyme Rx
38. L-glutathione Reduce
39. Glycine, L-gamma-glutamyl-l-cysteinyl-
40. Reduced L-glutathione
41. (2s)-2-amino-5-[[(2r)-1-(carboxymethylamino)-1-oxo-3-sulfanylpropan-2-yl]amino]-5-oxopentanoic Acid
42. (s)-2-amino-5-(((r)-1-((carboxymethyl)amino)-3-mercapto-1-oxopropan-2-yl)amino)-5-oxopentanoic Acid
43. N-(n-gamma-l-glutamyl-l-cysteinyl)glycine
44. N-(n-l-gamma-glutamyl-l-cysteinyl)glycine
45. Glycine, N-(n-l-gamma-glutamyl-l-cysteinyl)-
46. Gan16c9b8o
47. L-glutamyl-l-cysteinylglycine
48. L-glutathione (reduced Form)
49. Glycine, L-.gamma.-glutamyl-l-cysteinyl-
50. Benzenamine, 2-[(4-methoxyphenyl)methoxy]-
51. Chebi:16856
52. (2s)-2-amino-4-{[(1r)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic Acid
53. 106272-20-2
54. 95687-20-0
55. Dsstox_cid_3101
56. N-(n-l-.gamma.-glutamyl-l-cysteinyl)glycine
57. N5-((r)-1-((carboxymethyl)amino)-3-mercapto-1-oxopropan-2-yl)-l-glutamine
58. Dsstox_rid_76875
59. Dsstox_gsid_23101
60. [glu(-cys)]n-gly
61. Mfcd00065939
62. Cas-70-18-8
63. (s)-2-amino-5-((r)-1-(carboxymethylamino)-3-mercapto-1-oxopropan-2-ylamino)-5-oxopentanoic Acid
64. Glutathione, Reduced
65. Ccris 2094
66. Sr-05000002567
67. Glutathione [ban:jan]
68. Einecs 200-725-4
69. L-glutathione Reduced Form
70. Unii-gan16c9b8o
71. Nsc 400639
72. Phytochelatins
73. Readisorb
74. Glutathione;
75. Nsc400639
76. 1lbk
77. Ncgc00094976-01
78. Tathion (tn)
79. Glutathione (jp17)
80. Spectrum_000419
81. 1oe7
82. 1oe8
83. 1r4w
84. C(n-.gamma.glu-)g
85. Glutathione [ii]
86. Glutathione [mi]
87. Reduced Glutathione,(s)
88. Spectrum2_001500
89. Spectrum3_000946
90. Spectrum4_001056
91. Spectrum5_000940
92. Glutathione, Reduced Form
93. Glutathione [inci]
94. Bmse000185
95. Bmse000952
96. Bmse000956
97. Glutathione (reduced Type)
98. Glutathione [vandf]
99. Cys(n-.gamma.glu-)-gly
100. Schembl9167
101. Chembl1543
102. Glutathione [mart.]
103. Glutathione [usp-rs]
104. Glutathione [who-dd]
105. Kbiogr_001352
106. Kbioss_000899
107. Mls001333069
108. Divk1c_000075
109. Spectrum1502248
110. Spbio_001519
111. Gamma-glutamyl-cysteinyl-glycine
112. Gtpl6737
113. Dtxsid6023101
114. L-?-glutamyl-l-cysteinylglycine
115. L-glutathione Reduced, 97.0%
116. Chebi:60836
117. Hms500d17
118. Kbio1_000075
119. Kbio2_000899
120. Kbio2_003467
121. Kbio2_006035
122. Kbio3_002012
123. (gamma-glutamylcysteine)n-glycine
124. L-g-glutamyl-l-cysteinyl-glycine
125. Y-l-glutamyl-l-cysteinyl-glycine
126. L-?-glutamyl-l-cysteinyl-glycine
127. Ninds_000075
128. Glutathione [ep Monograph]
129. Hms1921n22
130. Pharmakon1600-01502248
131. Poly(gamma-glutamylcysteine)glycine
132. Hy-d0187
133. L-glutathione Reduced, >=98.0%
134. Zinc3830891
135. Gam.-l-glutamyl-l-cysteinyl-glycine
136. Tox21_111371
137. Bdbm50422268
138. Ccg-38876
139. L-gamma-glutamyl-l-cysteinyl-glycine
140. Nsc758199
141. S4606
142. Akos015999135
143. Tox21_111371_1
144. Cs-7948
145. Db00143
146. Nsc-758199
147. Sdccgmls-0066687.p001
148. .gamma.-l-glutamyl-l-cysteinyl-glycine
149. Idi1_000075
150. N-(n-l-?-glutamyl-l-cysteinyl)glycine
151. Pharm Biol 11: 539 (1968)
152. Smp1_000247
153. Ncgc00264046-02
154. Ds-14675
155. Gsh;gamma-l-glutamyl-l-cysteinyl-glycine
156. Smr000857220
157. Sbi-0051743.p002
158. L-glutathione Reduced, Bioxtra, >=98.0%
159. B7775
160. G0074
161. Glycine, N-(n-l-gamma-glutamyl-l-cysteinyl)
162. C00051
163. C02471
164. D00014
165. G-3980
166. P19615
167. Ab00443568_03
168. Glutathione 100 Microg/ml In Acetonitrile:water
169. A866658
170. Q116907
171. Sr-05000002567-1
172. Sr-05000002567-2
173. L-glutathione Reduced, Vetec(tm) Reagent Grade, >=98%
174. Z2183947556
175. Glutathione, European Pharmacopoeia (ep) Reference Standard
176. Glutathione, United States Pharmacopeia (usp) Reference Standard
177. Glutathione, Pharmaceutical Secondary Standard; Certified Reference Material
178. L-glutathione Reduced, Cell Culture Tested, Bioreagent, >=98.0%, Powder
179. (2s)-2-amino-4-{[(1r)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoylbutanoic Acid
180. (2s)-2-amino-5-({(2r)-1-[(carboxymethyl)amino]-1-oxo-3-sulfanyl-2-propanyl}amino)-5-oxopentanoic Acid
181. Glutathione; L-glutathione Reduced; 5-l-glutamyl-l-cysteinylglycine; Gamma-l-glutamyl-l-cysteinylglycine; Gsh
| Molecular Weight | 307.33 g/mol |
|---|---|
| Molecular Formula | C10H17N3O6S |
| XLogP3 | -4.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 307.08380644 g/mol |
| Monoisotopic Mass | 307.08380644 g/mol |
| Topological Polar Surface Area | 160 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 389 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For nutritional supplementation, also for treating dietary shortage or imbalance
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB32 - Glutathione
Absorption
Research suggests that glutathione is not orally bioactive, and that very little of oral glutathione tablets or capsules is actually absorbed by the body.
Glutathione (GSH) participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase. It also plays a role in the hepatic biotransformation and detoxification process; it acts as a hydrophilic molecule that is added to other lipophilic toxins or wastes prior to entering biliary excretion. It participates in the detoxification of methylglyoxal, a toxic by-product of metabolism, mediated by glyoxalase enzymes. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. GSH is a cofactor of conjugation and reduction reactions that are catalyzed by glutathione S-transferase enzymes expressed in the cytosol, microsomes, and mitochondria. However, it is capable of participating in non-enzymatic conjugation with some chemicals, as it is hypothesized to do to a significant extent with n-acetyl-p-benzoquinone imine (NAPQI), the reactive cytochrome P450 reactive metabolite formed by toxic overdose of acetaminophen. Glutathione in this capacity binds to NAPQI as a suicide substrate and in the process detoxifies it, taking the place of cellular protein sulfhydryl groups which would otherwise be toxically adducted. The preferred medical treatment to an overdose of this nature, whose efficacy has been consistently supported in literature, is the administration (usually in atomized form) of N-acetylcysteine, which is used by cells to replace spent GSSG and allow a usable GSH pool.