

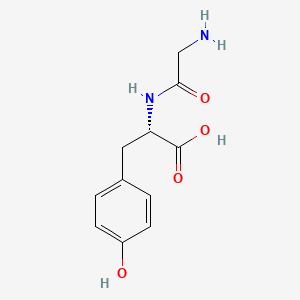

1. Gly-tyr
2. Glycyl-tyrosine
3. Glycyltyrosine
1. 658-79-7
2. Gly-tyr
3. N-glycyl-l-tyrosine
4. H-gly-tyr-oh
5. Glycyltyrosine
6. L-tyrosine, Glycyl-
7. Glycyl-tyrosine
8. (s)-2-(2-aminoacetamido)-3-(4-hydroxyphenyl)propanoic Acid
9. Glycyl-l-tyrosine 2-hydrate
10. Chembl53400
11. Chebi:73517
12. (2s)-2-[(2-aminoacetyl)amino]-3-(4-hydroxyphenyl)propanoic Acid
13. A226496h4o
14. L-tyrosine, Glycyl-, Monomer
15. Gy Dipeptide
16. Nsc-83260
17. Unii-a226496h4o
18. G-y Dipeptide
19. Einecs 211-525-1
20. Gly-l-tyr
21. Nsc 83260
22. Gly-l-tyr-oh
23. L-glycyl-l-tyrosine
24. Nsc 118362
25. Glycine Tyrosine Dipeptide
26. Glycine-tyrosine Dipeptide
27. Schembl479126
28. Glycyltyrosine [who-dd]
29. (2s)-2-(2-aminoacetamido)-3-(4-hydroxyphenyl)propanoic Acid
30. Dtxsid701316223
31. Bcp24671
32. Glycyl-l-tyrosine [usp-rs]
33. Gy
34. Zinc1730678
35. Bdbm50188508
36. Stl466189
37. Akos010366204
38. Ac-6976
39. Cs-w010308
40. Hy-w009592
41. S10390
42. Ds-13668
43. G0145
44. G-6370
45. 658g797
46. Q27140599
47. 2-(2-amino-acetylamino)-3-(4-hydroxy-phenyl)-propionic Acid
48. (s)-2-(2-amino-acetylamino)-3-(4-hydroxy-phenyl)-propionic Acid
49. G-y
50. Melanin Synthesized From Gly-tyr Substrate Catalyzed By Tyrosinase For 22 Hrs, >10 Kd Fraction
51. Melanin Synthesized From Gly-tyr Substrate Catalyzed By Tyrosinase For 3 Hrs, Hcl Insoluble Portion (precipitate)
52. Melanin Synthesized From Gly-tyr Substrate Catalyzed By Tyrosinase, Brominated With N-bromosuccinimide
53. Melanin Synthesized From Gly-tyr Substrate Catalyzed By Tyrosinase, Sulfonated Using Sulfur Trioxide/dmf Complex For 1.5-7 Hours
| Molecular Weight | 238.24 g/mol |
|---|---|
| Molecular Formula | C11H14N2O4 |
| XLogP3 | -3.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 238.09535693 g/mol |
| Monoisotopic Mass | 238.09535693 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 275 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |