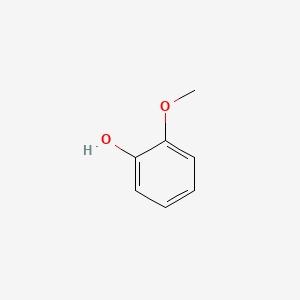

1. 2-hydroxy-anisole
2. 2-hydroxyanisole
3. 2-methoxy-phenol
4. 2-methoxyphenol
5. Catechol, Methyl
6. Guaicol
7. Methyl Catechol
1. 2-methoxyphenol
2. 90-05-1
3. O-methoxyphenol
4. 2-hydroxyanisole
5. Phenol, 2-methoxy-
6. Guaiastil
7. Pyroguaiac Acid
8. O-guaiacol
9. O-hydroxyanisole
10. Pyrocatechol Monomethyl Ether
11. 1-hydroxy-2-methoxybenzene
12. Methylcatechol
13. Anastil
14. Guaicol
15. Phenol, O-methoxy-
16. Guaicolina
17. Guajol
18. Guasol
19. O-methyl Catechol
20. Catechol Monomethyl Ether
21. Creosote, Wood
22. Methoxyphenol
23. Guajakol
24. Creodon
25. 8021-39-4
26. Wood Creosote
27. 2-methoxy-phenol
28. Hydroxyanisole
29. Fema No. 2532
30. Guaiacol [jan]
31. Methylcatachol
32. Ortho-guaiacol
33. 2-methoxy Phenol
34. Nsc 3815
35. (mu)-methoxyphenol
36. Mfcd00002185
37. Guaiacol (jan)
38. Creodon (tn)
39. Nsc-3815
40. 2-methoxyl-4-vinylphenol
41. 6jka7mah9c
42. 9009-62-5
43. 2-methoxyphenol (guaiacol)
44. Chembl13766
45. Chebi:28591
46. Phenol, 2-methoxy-, Homopolymer
47. Phenol, Methoxy-
48. Ncgc00090827-02
49. Ncgc00090827-04
50. Guajacol
51. Dsstox_cid_3113
52. Dsstox_rid_76880
53. Guajakol [czech]
54. Dsstox_gsid_23113
55. Creosote, Beechwood
56. Guaiacol (natural)
57. Pyrocatechol Methyl Ester
58. Cas-90-05-1
59. 26247-00-7
60. Ccris 2943
61. Guaiacol [jan:nf]
62. Hsdb 4241
63. Sr-01000838056
64. Einecs 201-964-7
65. Unii-6jka7mah9c
66. Guiacol
67. Creasote
68. Methoxy Phenol
69. 6-methoxyphenol
70. Hydroxyl Anisole
71. Ai3-05615
72. Nat.guaiacol
73. O-methylcatechol
74. O-guiacol
75. O--methoxyphenol
76. Orthomethoxyphenol
77. O-methoxy-phenol
78. 2-methyloxyphenol
79. Ortho-methoxyphenol
80. Guaiacol,(s)
81. Jz3
82. 2-(methyloxy)phenol
83. Guaiacol [fhfi]
84. Guaiacol [hsdb]
85. Guaiacol [mi]
86. Guaiacol [vandf]
87. Catechol Mono Methyl Ether
88. Guaiacol [mart.]
89. Bmse000436
90. Bmse010027
91. Guaiacol [usp-rs]
92. Guaiacol [who-dd]
93. Ec 201-964-7
94. Guaiacol, Puriss., 99%
95. Wln: Qr Bo1
96. Dsstox_rid_77552
97. 1- Hydroxy-2-methoxybenzene
98. 3-methoxy-4-hydroxy Benzene
99. Dsstox_gsid_24853
100. Schembl21626
101. Ghl.pd_mitscher_leg0.900
102. Guaiacol (liquid) Extra Pure
103. Guaiacol, Oxidation Indicator
104. Mls001055375
105. Guaiacol [ep Monograph]
106. Guaiacol [usp Impurity]
107. Dtxsid0023113
108. Nsc3815
109. Guaiacol, Natural, >=99%, Fg
110. Hms2089d18
111. Hms2233p04
112. Hms3372n11
113. Hms3715e11
114. Pharmakon1600-01506165
115. A Hydroxlyated Aryl Lignin Fragment
116. Bcp27082
117. Cs-d1347
118. Hy-n1380
119. Str03604
120. Tox21_111031
121. Tox21_201136
122. Tox21_202990
123. Tox21_400004
124. Bdbm50240369
125. Nsc760376
126. S3872
127. Stl281868
128. Zinc13512224
129. Akos000118831
130. Ccg-214035
131. Db11359
132. Nsc-760376
133. Pb43791
134. Ps-3252
135. Guaiacol, Saj First Grade, >=98.0%
136. Ncgc00090827-01
137. Ncgc00090827-03
138. Ncgc00090827-05
139. Ncgc00090827-06
140. Ncgc00090827-07
141. Ncgc00258688-01
142. Ncgc00260535-01
143. Ac-34997
144. Guaiacol, Vetec(tm) Reagent Grade, 98%
145. Smr000059155
146. Sy048708
147. Cas-8021-39-4
148. Db-024854
149. Ft-0626815
150. Ft-0671312
151. Guaifenesin Impurity A [ep Impurity]
152. M0121
153. C01502
154. D00117
155. F70227
156. 2-methoxyphenol;o-methoxyphenol;2-hydroxyanisole
157. Ab00876226-06
158. Ab00876226_07
159. A843426
160. Q412403
161. Q-100002
162. Sr-01000838056-2
163. Sr-01000838056-3
164. F2173-0425
165. Guaiacol, European Pharmacopoeia (ep) Reference Standard
166. Guaiacol, United States Pharmacopeia (usp) Reference Standard
167. Guaiacol, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 124.14 g/mol |
|---|---|
| Molecular Formula | C7H8O2 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 124.052429494 g/mol |
| Monoisotopic Mass | 124.052429494 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 83 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
IT IS USED EMPIRICALLY AS AN EXPECTORANT TO LESSEN AMT OF MUCOUS IN THE CHRONIC STAGES OF BRONCHITIS & BRONCHIECTASIS. IT IS FREQUENTLY PRESCRIBED FOR ITS STIMULANT EXPECTORANT ACTION AS A CONSTITUENT OF A STEAM INHALANT. THE ANTISEPTIC ACTION IS WEAK & NOT CLINICALLY USEFUL. ... IT HAS BEEN EMPLOYED AS A LOCAL ANESTHETIC IN DENTISTRY, IN A MANNER SIMILAR TO THYMOL. IT IS OCCASIONALLY EMPLOYED EXTERNALLY FOR ITS DISINFECTANT ACTION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1102
MEDICATION (VET): TOPICALLY (1.0-1.5%), IN UDDER AND WOUND TREATMENT OINTMENTS & IN POULTICES; WHEN ELIMINATED BY RESP MUCOUS MEMBRANES, SLIGHT ANTISEPTIC & LOCAL ANESTHETIC EFFECTS MAY OCCUR. INTERNALLY ... USED IN ANTIFERMENT & ANTIBLOAT MIXTURES.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 144
ANTIPYRETIC; STYPTIC; ASTRINGENT
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2601
MEDICATION (VET): PARASITICIDE, DEODORANT
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 403
Disinfectants; Expectorants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Medication (Vet): ... Oral or parenteral mucolytic antiseptic antitussive in bronchopneumonias. ... Inhalant. In antiphlogistic, anodyne liquids on congested udders, in horse leg paints, and in poultices. Spray over tail biting swine to ... discourage cannibalism. Parenteral /dosage/ in camphorated oil. US mfr use 50 mg/mL concentration.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 249
For the symptomatic relief of coughs associated with colds, bronchial catarrh, influenza and upper respiratory tract infections such as laryngitis and pharyngitis. Codeine is a well-known centrally acting cough suppressant. Guaiacol acts as an expectorant, loosening bronchial secretions in the respiratory tract. /Guaiacol, codeine combination/
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Pulmo Bailly (Last updated February 2010). Available from, as of March 9, 2010: https://emc.medicines.org.uk/medicine/22147/SPC/Pulmo+Bailly/
THE REPEATED ABSORPTION OF THERAPEUTIC DOSES FROM GASTROENTERIC TRACT MAY INDUCE SIGNS OF CHRONIC INTOXICATION, CHARACTERIZED BY DISTURBANCES OF VISION & DIGESTION (INCR PERISTALSIS & EXCRETION OF BODY FECES). IN ISOLATED CASES OF "SELF-MEDICATION", HYPERTENSION & ALSO GENERAL CARDIOVASCULAR COLLAPSE HAVE BEEN DESCRIBED.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2603
WHEREVER CREOSOTE IS INDICATED FOR INTERNAL MEDICATION CREOSOTE FROM WOOD TAR SHOULD BE DISPENSED & UNDER NO CIRCUMSTANCES SHOULD CREOSOTE FROM COAL TAR BE GIVEN, UNLESS EXPLICITLY SO DIRECTED.
The Merck Index. 10th ed. Rahway, New Jersey: Merck Co., Inc., 1983., p. 368
Do not use in Cats.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 403
It is used medicinally as an expectorant, antiseptic, and local anesthetic. Guaiacol is used in traditional dental pulp sedation, and has the property of inducing cell proliferation; guaiacol is a potent scavenger of reactive oxygen radicals and its radical scavenging activity may be associated with its effect on cell proliferation.
Absorption
In rats, guaiacol is rapidly absorbed, being present in the blood 5 minutes after oral administration, and reaching its peak plasma concentration in about 10 minutes. Its elimination from the blood is usually as rapid.
Route of Elimination
Excreted by rabbits in combined form with sulfate (15%) and glucuronic acid (72%).
CREOSOTE IS RAPIDLY ABSORBED FROM THE GASTROENTERIC TRACT AND THROUGH THE SKIN. IT APPEARS TO BE EXCRETED IN THE URINE MAINLY IN CONJUGATION WITH SULFURIC, HEXURONIC, & OTHER ACIDS. OXIDATION ALSO OCCURS WITH THE FORMATION OF CMPD THAT IMPART A SMOKY APPEARANCE TO THE URINE. TRACES ARE EXCRETED BY WAY OF THE LUNGS.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2603
Medical experience indicates that toxic quantities can be absorbed through the skin quite readily.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2525
Methoxyphenol largely absorbed from digestive tract and stored in blood, kidneys, and respiratory organs. Excreted by rabbits in combined form with sulfate (15%) and glucuronic acid (72%).
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 740
In rats, guaiacol is rapidly absorbed, being present in the blood 5 minutes after oral administration, and reaching its peak plasma concentration in about 10 minutes. Its elimination from the blood is usually as rapid.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Pulmo Bailly (Last updated February 2010). Available from, as of March 9, 2010: https://emc.medicines.org.uk/medicine/22147/SPC/Pulmo+Bailly/
.../IT APPEARS TO BE CONJUGATED/ WITH SULFURIC, HEXURONIC & OTHER ACIDS. OXIDATION...OCCURS WITH FORMATION OF COMPOUNDS THAT IMPART A SMOKY APPEARANCE TO THE URINE.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2603
Several strains of Aspergillus niger hydroxylated anisole to give o-hydroxyanisole as main product.
Bocks SM; Phytochemistry 6 (6): 785 (1967)
o-Methoxyphenol yields 3-methoxycatechol probably in rabbit; yields o-methoxyphenyl sulfate probably in rabbit. o-Methoxyphenol yields catechol in rat; yields 1,2-dimethoxybenzene in mouse, rabbit, guinea pig, and rat. /From table/
SRI